J Periodontal Implant Sci.
2016 Oct;46(5):291-302. 10.5051/jpis.2016.46.5.291.
Benefits of mineralized bone cortical allograft for immediate implant placement in extraction sites: an in vivo study in dogs
- Affiliations
-
- 1Department of Periodontology, Montpellier University Hospital, Montpellier, France. orti.valerie.30@gmail.com
- 2Laboratory of Bioengineering and Nanoscience, University of Montpellier, Montpellier, France.
- 3Department of Stomatology, Centro Ciencias de la Salud, Universidad Autónoma, Aguascalientes, Mexico.
- KMID: 2354928
- DOI: http://doi.org/10.5051/jpis.2016.46.5.291
Abstract
- PURPOSE
The aim of the present study was to evaluate the effectiveness of using a mineralized bone cortical allograft (MBCA), with or without a resorbable collagenous membrane derived from bovine pericardium, on alveolar bone remodeling after immediate implant placement in a dog model.
METHODS
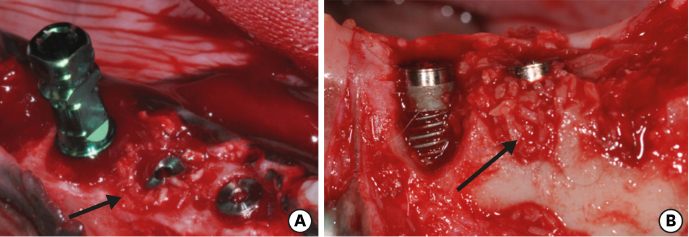
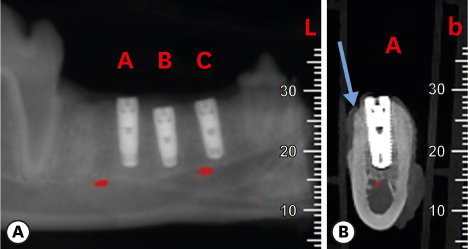
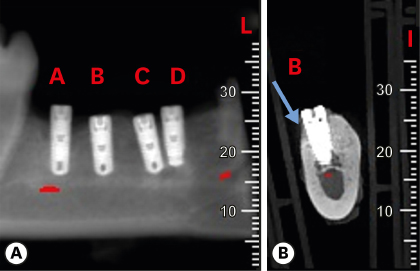
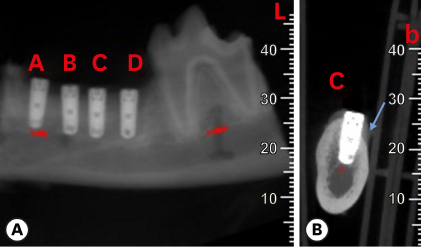
Six mongrel dogs were included. The test and control sites were randomly selected. Four biradicular premolars were extracted from the mandible. In control sites, implants without an allograft or membrane were placed immediately in the fresh extraction sockets. In the test sites, an MBCA was placed to fill the gap between the bone socket wall and implant, with or without a resorbable collagenous membrane. Specimens were collected after 1 and 3 months. The amount of residual particles and new bone quality were evaluated by histomorphometry.
RESULTS
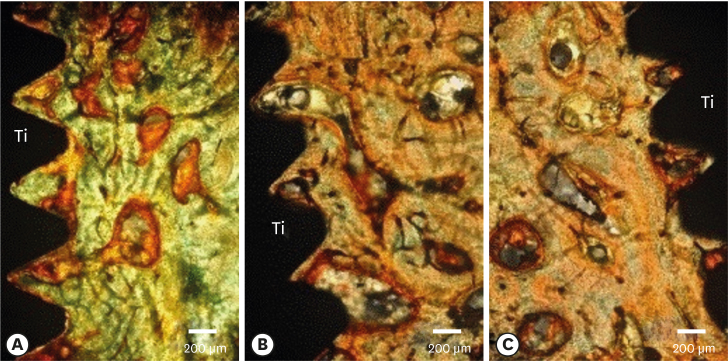
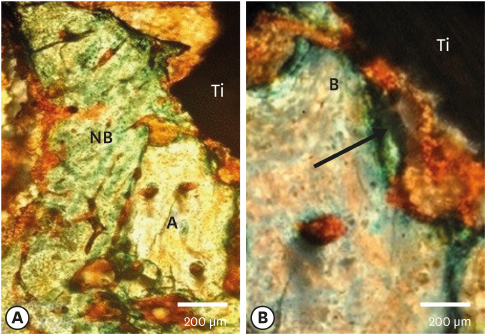
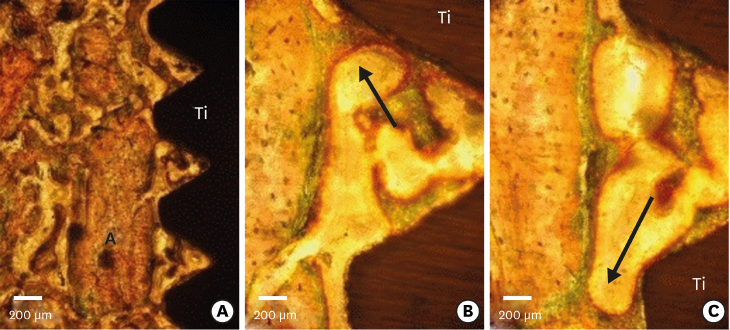
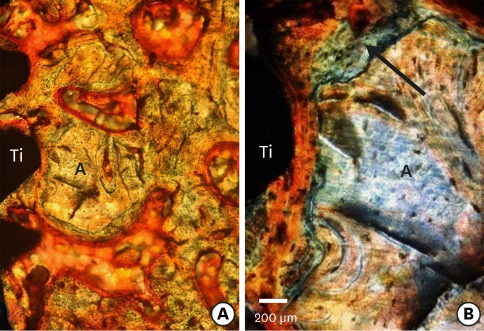
Few residual graft particles were observed to be closely embedded in the new bone without any contact with the implant surface. The allograft combined with a resorbable collagen membrane limited the resorption of the buccal wall in height and width. The histological quality of the new bone was equivalent to that of the original bone. The MBCA improved the quality of new bone formation, with few residual particles observed at 3 months.
CONCLUSIONS
The preliminary results of this animal study indicate a real benefit in obtaining new bone as well as in enhancing osseointegration due to the high resorbability of cortical allograft particles, in comparison to the results of xenografts or other biomaterials (mineralized or demineralized cancellous allografts) that have been presented in the literature. Furthermore, the use of an MBCA combined with a collagen membrane in extraction and immediate implant placement limited the extent of post-extraction resorption.
MeSH Terms
Figure
Reference
-
1. Wheeler SL, Vogel RE, Casellini R. Tissue preservation and maintenance of optimum esthetics: a clinical report. Int J Oral Maxillofac Implants. 2000; 15:265–271.2. Paolantonio M, Dolci M, Scarano A, d’Archivio D, di Placido G, Tumini V, et al. Immediate implantation in fresh extraction sockets. A controlled clinical and histological study in man. J Periodontol. 2001; 72:1560–1571.
Article3. Chen ST, Wilson TG Jr, Hämmerle CH. Immediate or early placement of implants following tooth extraction: review of biologic basis, clinical procedures, and outcomes. Int J Oral Maxillofac Implants. 2004; 19:Suppl. 12–25.4. Lindeboom JA, Tjiook Y, Kroon FH. Immediate placement of implants in periapical infected sites: a prospective randomized study in 50 patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006; 101:705–710.
Article5. Schropp L, Isidor F, Kostopoulos L, Wenzel A. Patient experience of, and satisfaction with, delayed-immediate vs. delayed single-tooth implant placement. Clin Oral Implants Res. 2004; 15:498–503.
Article6. Araújo MG, Lindhe J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J Clin Periodontol. 2005; 32:212–218.
Article7. Araújo MG, Sukekava F, Wennström JL, Lindhe J. Tissue modeling following implant placement in fresh extraction sockets. Clin Oral Implants Res. 2006; 17:615–624.
Article8. Araújo MG, Wennström JL, Lindhe J. Modeling of the buccal and lingual bone walls of fresh extraction sites following implant installation. Clin Oral Implants Res. 2006; 17:606–614.
Article9. Tan WL, Wong TL, Wong MC, Lang NP. A systematic review of post-extractional alveolar hard and soft tissue dimensional changes in humans. Clin Oral Implants Res. 2012; 23:Suppl 5. 1–21.
Article10. Simion M, Dahlin C, Trisi P, Piattelli A. Qualitative and quantitative comparative study on different filling materials used in bone tissue regeneration: a controlled clinical study. Int J Periodontics Restorative Dent. 1994; 14:198–215.11. Chen ST, Darby IB, Adams GG, Reynolds EC. A prospective clinical study of bone augmentation techniques at immediate implants. Clin Oral Implants Res. 2005; 16:176–184.
Article12. Barone A, Ricci M, Calvo-Guirado JL, Covani U. Bone remodelling after regenerative procedures around implants placed in fresh extraction sockets: an experimental study in Beagle dogs. Clin Oral Implants Res. 2011; 22:1131–1137.
Article13. Dimova C. Socket preservation procedure after tooth extraction. Key Eng Mater. 2014; 587:325–330.
Article14. Evans CD, Chen ST. Esthetic outcomes of immediate implant placements. Clin Oral Implants Res. 2008; 19:73–80.
Article15. Han JY, Shin SI, Herr Y, Kwon YH, Chung JH. The effects of bone grafting material and a collagen membrane in the ridge splitting technique: an experimental study in dogs. Clin Oral Implants Res. 2011; 22:1391–1398.
Article16. Iasella JM, Greenwell H, Miller RL, Hill M, Drisko C, Bohra AA, et al. Ridge preservation with freeze-dried bone allograft and a collagen membrane compared to extraction alone for implant site development: a clinical and histologic study in humans. J Periodontol. 2003; 74:990–999.
Article17. Simon BI, Von Hagen S, Deasy MJ, Faldu M, Resnansky D. Changes in alveolar bone height and width following ridge augmentation using bone graft and membranes. J Periodontol. 2000; 71:1774–1791.
Article18. Vignoletti F, Sanz M. Immediate implants at fresh extraction sockets: from myth to reality. Periodontol 2000. 2014; 66:132–152.
Article19. Feuille F, Knapp CI, Brunsvold MA, Mellonig JT. Clinical and histologic evaluation of bone-replacement grafts in the treatment of localized alveolar ridge defects. Part 1: mineralized freeze-dried bone allograft. Int J Periodontics Restorative Dent. 2003; 23:29–35.20. Tomasi C, Sanz M, Cecchinato D, Pjetursson B, Ferrus J, Lang NP, et al. Bone dimensional variations at implants placed in fresh extraction sockets: a multilevel multivariate analysis. Clin Oral Implants Res. 2010; 21:30–36.
Article21. Qahash M, Susin C, Polimeni G, Hall J, Wikesjö UM. Bone healing dynamics at buccal peri-implant sites. Clin Oral Implants Res. 2008; 19:166–172.
Article22. Botticelli D, Berglundh T, Buser D, Lindhe J. The jumping distance revisited: an experimental study in the dog. Clin Oral Implants Res. 2003; 14:35–42.23. Botticelli D, Renzi A, Lindhe J, Berglundh T. Implants in fresh extraction sockets: a prospective 5-year follow-up clinical study. Clin Oral Implants Res. 2008; 19:1226–1232.
Article24. Wilson TG Jr, Schenk R, Buser D, Cochran D. Implants placed in immediate extraction sites: a report of histologic and histometric analyses of human biopsies. Int J Oral Maxillofac Implants. 1998; 13:333–341.25. Botticelli D, Berglundh T, Lindhe J. Hard-tissue alterations following immediate implant placement in extraction sites. J Clin Periodontol. 2004; 31:820–828.
Article26. Klinge B, Alberius P, Isaksson S, Jönsson J. Osseous response to implanted natural bone mineral and synthetic hydroxylapatite ceramic in the repair of experimental skull bone defects. J Oral Maxillofac Surg. 1992; 50:241–249.
Article27. Araújo M, Linder E, Wennström J, Lindhe J. The influence of Bio-Oss Collagen on healing of an extraction socket: an experimental study in the dog. Int J Periodontics Restorative Dent. 2008; 28:123–135.28. Becker W. Treatment of small defects adjacent to oral implants with various biomaterials. Periodontol 2000. 2003; 33:26–35.
Article29. Block MS, Finger I, Lytle R. Human mineralized bone in extraction sites before implant placement: preliminary results. J Am Dent Assoc. 2002; 133:1631–1638.30. Brugnami F, Then PR, Moroi H, Leone CW. Histologic evaluation of human extraction sockets treated with demineralized freeze-dried bone allograft (DFDBA) and cell occlusive membrane. J Periodontol. 1996; 67:821–825.
Article31. Keith JD Jr, Petrungaro P, Leonetti JA, Elwell CW, Zeren KJ, Caputo C, et al. Clinical and histologic evaluation of a mineralized block allograft: results from the developmental period (2001–2004). Int J Periodontics Restorative Dent. 2006; 26:321–327.32. Lee DW, Pi SH, Lee SK, Kim EC. Comparative histomorphometric analysis of extraction sockets healing implanted with bovine xenografts, irradiated cancellous allografts, and solvent-dehydrated allografts in humans. Int J Oral Maxillofac Implants. 2009; 24:609–615.33. Schropp L, Kostopoulos L, Wenzel A. Bone healing following immediate versus delayed placement of titanium implants into extraction sockets: a prospective clinical study. Int J Oral Maxillofac Implants. 2003; 18:189–199.34. Simion M, Trisi P, Piattelli A. GBR with an e-PTFE membrane associated with DFDBA: histologic and histochemical analysis in a human implant retrieved after 4 years of loading. Int J Periodontics Restorative Dent. 1996; 16:338–347.35. Wang HL, Tsao YP. Histologic evaluation of socket augmentation with mineralized human allograft. Int J Periodontics Restorative Dent. 2008; 28:231–237.36. Wood RA, Mealey BL. Histologic comparison of healing after tooth extraction with ridge preservation using mineralized versus demineralized freeze-dried bone allograft. J Periodontol. 2012; 83:329–336.
Article37. Wang HL, Boyapati L. “PASS” principles for predictable bone regeneration. Implant Dent. 2006; 15:8–17.
Article38. Hämmerle CH, Jung RE, Yaman D, Lang NP. Ridge augmentation by applying bioresorbable membranes and deproteinized bovine bone mineral: a report of twelve consecutive cases. Clin Oral Implants Res. 2008; 19:19–25.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Guided Bone Regeneration Using Mineralized Bone Allograft and Barrier Membrane Derived from Ox Pericardium
- Clinical Evaluation about the Immediate Implant Replacement after Tooth Extraction
- The effect of non-resorbable barrier membrane on the change of buccal and lingual alveolar bone in immediate implant placement into periapically infected extraction sockets
- A STUDY OF BONE APPOSITION AND MARGINAL ALVEOLAR BONE LOSS AROUND IMMEDIATE IMPLANSTS
- Regeneration of total tissue using alveolar ridge augmentation with soft tissue substitute on periodontally compromised extraction sites: case report