Yonsei Med J.
2015 Nov;56(6):1671-1677. 10.3349/ymj.2015.56.6.1671.
The Effects of Two Non-Steroidal Anti-Inflammatory Drugs, Bromfenac 0.1% and Ketorolac 0.45%, on Cataract Surgery
- Affiliations
-
- 1Department of Ophthalmology, Severance Hospital, Corneal Dystrophy Research Institute, Yonsei University College of Medicine, Seoul, Korea. tikim@yuhs.ac
- 2Department of Ophthalmology and Inha Vision Science Laboratory, Inha University School of Medicine, Incheon, Korea.
- 3Institute of Vision Research, Severance Biomedical Science Institute, Brain Korea 21 Plus Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2345899
- DOI: http://doi.org/10.3349/ymj.2015.56.6.1671
Abstract
- PURPOSE
To compare the additive effects of two types of non-steroidal anti-inflammatory drugs (NSAIDs), bromfenac 0.1% or ketorolac 0.45%, relative to topical steroid alone in cataract surgery.
MATERIALS AND METHODS
A total 91 subjects scheduled to undergo cataract operation were randomized into three groups: Group 1, pre/postoperative bromfenac 0.1%; Group 2, pre/postoperative preservative-free ketorolac 0.45%; and Group 3, postoperative steroid only, as a control. Outcome measures included intraoperative change in pupil size, postoperative anterior chamber inflammation control, change in macular thickness and volume, and ocular surface status after operation.
RESULTS
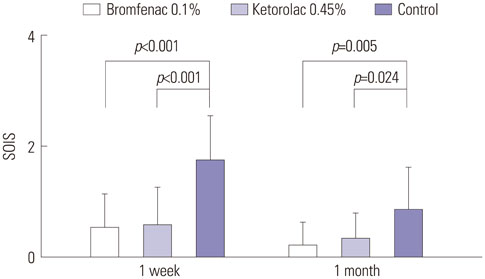
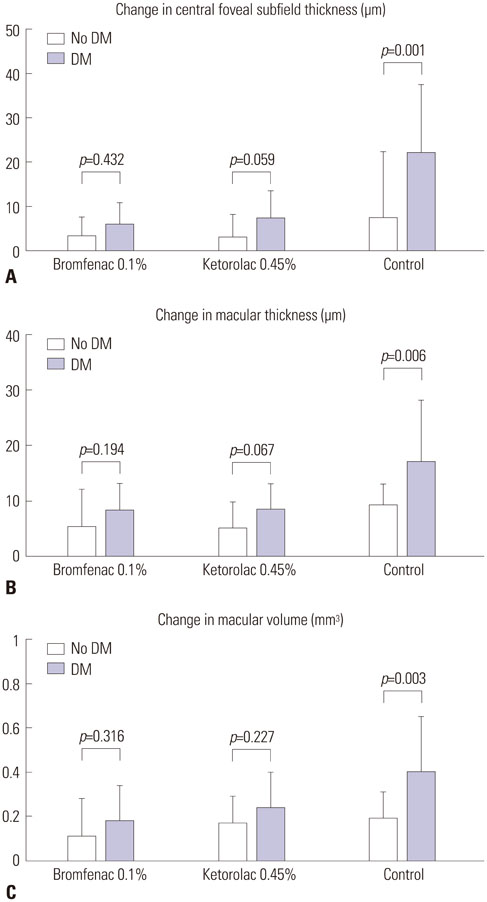
Both NSAID groups had smaller intraoperative pupil diameter changes compared to the control group (p<0.05). There was significantly less ocular inflammation 1 week and 1 month postoperatively in both NSAID groups than the control group. The changes in central foveal subfield thickness measured before the operation and at postoperative 1 month were 4.30+/-4.25, 4.87+/-6.03, and 12.47+/-12.24 microm in groups 1 to 3, respectively. In the control group, macular thickness and volume increased more in patients with diabetes mellitus (DM), compared to those without DM. In contrast, in both NSAID groups, NSAIDs significantly reduced macular changes in subgroups of patients with or without DM. Although three ocular surface parameters were worse in group 1 than in group 2, these differences were not significant.
CONCLUSION
Adding preoperative and postoperative bromfenac 0.1% or ketorolac 0.45% to topical steroid can reduce intraoperative miosis, postoperative inflammation, and macular changes more effectively than postoperative steroid alone.
MeSH Terms
-
Aged
Anti-Inflammatory Agents, Non-Steroidal/*administration & dosage/pharmacology
Benzophenones/*administration & dosage/pharmacology
Bromobenzenes/*administration & dosage/pharmacology
*Cataract
*Cataract Extraction
Female
Humans
Inflammation/prevention & control
Ketorolac/*administration & dosage/pharmacology
Lens Implantation, Intraocular
Macular Edema/*prevention & control
Male
Middle Aged
Miosis/*prevention & control
Phacoemulsification
Postoperative Complications/drug therapy
Postoperative Period
Premedication
Treatment Outcome
Anti-Inflammatory Agents, Non-Steroidal
Benzophenones
Bromobenzenes
Ketorolac
Figure
Reference
-
1. Ahuja M, Dhake AS, Sharma SK, Majumdar DK. Topical ocular delivery of NSAIDs. AAPS J. 2008; 10:229–241.2. Donnenfeld ED, Donnenfeld A. Global experience with Xibrom (bromfenac ophthalmic solution) 0.09%: the first twice-daily ophthalmic nonsteroidal anti-inflammatory drug. Int Ophthalmol Clin. 2006; 46:21–40.
Article3. Henderson BA, Gayton JL, Chandler SP, Gow JA, Klier SM, McNamara TR, et al. Safety and efficacy of bromfenac ophthalmic solution (Bromday) dosed once daily for postoperative ocular inflammation and pain. Ophthalmology. 2011; 118:2120–2127.
Article4. Silverstein SM, Cable MG, Sadri E, Peace JH, Fong R, Chandler SP, et al. Once daily dosing of bromfenac ophthalmic solution 0.09% for postoperative ocular inflammation and pain. Curr Med Res Opin. 2011; 27:1693–1703.
Article5. Cho H, Wolf KJ, Wolf EJ. Management of ocular inflammation and pain following cataract surgery: focus on bromfenac ophthalmic solution. Clin Ophthalmol. 2009; 3:199–210.
Article6. McColgin AZ, Heier JS. Control of intraocular inflammation associated with cataract surgery. Curr Opin Ophthalmol. 2000; 11:3–6.
Article7. Perry HD, Donnenfeld ED. An update on the use of ophthalmic ketorolac tromethamine 0.4%. Expert Opin Pharmacother. 2006; 7:99–107.
Article8. McGhee CN, Dean S, Danesh-Meyer H. Locally administered ocular corticosteroids: benefits and risks. Drug Saf. 2002; 25:33–55.9. Heier JS, Topping TM, Baumann W, Dirks MS, Chern S. Ketorolac versus prednisolone versus combination therapy in the treatment of acute pseudophakic cystoid macular edema. Ophthalmology. 2000; 107:2034–2038.
Article10. Wolf EJ, Braunstein A, Shih C, Braunstein RE. Incidence of visually significant pseudophakic macular edema after uneventful phacoemulsification in patients treated with nepafenac. J Cataract Refract Surg. 2007; 33:1546–1549.
Article11. Donnenfeld ED, Nichamin LD, Hardten DR, Raizman MB, Trattler W, Rajpal RK, et al. Twice-daily, preservative-free ketorolac 0.45% for treatment of inflammation and pain after cataract surgery. Am J Ophthalmol. 2011; 151:420–426.
Article12. Guidera AC, Luchs JI, Udell IJ. Keratitis, ulceration, and perforation associated with topical nonsteroidal anti-inflammatory drugs. Ophthalmology. 2001; 108:936–944.
Article13. Flach AJ. Corneal melts associated with topically applied nonsteroidal anti-inflammatory drugs. Trans Am Ophthalmol Soc. 2001; 99:205–210.14. Yanai K, Huang J, Kadonosono K, Uchio E. Corneal sensitivity after topical bromfenac sodium eye-drop instillation. Clin Ophthalmol. 2013; 7:741–744.15. Xu K, McDermott M, Villanueva L, Schiffman RM, Hollander DA. Ex vivo corneal epithelial wound healing following exposure to ophthalmic nonsteroidal anti-inflammatory drugs. Clin Ophthalmol. 2011; 5:269–274.16. Donnenfeld ED, Holland EJ, Stewart RH, Gow JA, Grillone LR. Bromfenac Ophthalmic Solution 0.09% (Xibrom) Study Group. Bromfenac ophthalmic solution 0.09% (Xibrom) for postoperative ocular pain and inflammation. Ophthalmology. 2007; 114:1653–1662.
Article17. Early Treatment Diabetic Retinopathy Study design and baseline patient characteristics. ETDRS report number 7. Ophthalmology. 1991; 98:5 Suppl. 741–756.18. Kim SJ, Equi R, Bressler NM. Analysis of macular edema after cataract surgery in patients with diabetes using optical coherence tomography. Ophthalmology. 2007; 114:881–889.
Article19. Wittpenn JR, Silverstein S, Heier J, Kenyon KR, Hunkeler JD, Earl M, et al. A randomized, masked comparison of topical ketorolac 0.4% plus steroid vs steroid alone in low-risk cataract surgery patients. Am J Ophthalmol. 2008; 146:554–560.
Article20. Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003; 22:640–650.
Article21. Miyake K, Ogawa T, Tajika T, Gow JA, McNamara TR. Ocular pharmacokinetics of a single dose of bromfenac sodium ophthalmic solution 0.1% in human aqueous humor. J Ocul Pharmacol Ther. 2008; 24:573–578.22. Solomon KD, Turkalj JW, Whiteside SB, Stewart JA, Apple DJ. Topical 0.5% ketorolac vs 0.03% flurbiprofen for inhibition of miosis during cataract surgery. Arch Ophthalmol. 1997; 115:1119–1122.
Article23. Cervantes-Coste G, Sánchez-Castro YG, Orozco-Carroll M, Mendoza-Schuster E, Velasco-Barona C. Inhibition of surgically induced miosis and prevention of postoperative macular edema with nepafenac. Clin Ophthalmol. 2009; 3:219–226.24. Russo A, Costagliola C, Delcassi L, Parmeggiani F, Romano MR, Dell'Omo R, et al. Topical nonsteroidal anti-inflammatory drugs for macular edema. Mediators Inflamm. 2013; 2013:476525.
Article25. Menke MN, Dabov S, Knecht P, Sturm V. Reproducibility of retinal thickness measurements in healthy subjects using spectralis optical coherence tomography. Am J Ophthalmol. 2009; 147:467–472.
Article26. Qaum T, Xu Q, Joussen AM, Clemens MW, Qin W, Miyamoto K, et al. VEGF-initiated blood-retinal barrier breakdown in early diabetes. Invest Ophthalmol Vis Sci. 2001; 42:2408–2413.27. Quinn CJ. Cystoid macular edema. Optom Clin. 1996; 5:111–130.28. Bucci FA Jr, Waterbury LD. Comparison of ketorolac 0.4% and bromfenac 0.09% at trough dosing: aqueous drug absorption and prostaglandin E2 levels. J Cataract Refract Surg. 2008; 34:1509–1512.
Article29. Waterbury LD, Silliman D, Jolas T. Comparison of cyclooxygenase inhibitory activity and ocular anti-inflammatory effects of ketorolac tromethamine and bromfenac sodium. Curr Med Res Opin. 2006; 22:1133–1140.
Article30. Walters T, Raizman M, Ernest P, Gayton J, Lehmann R. In vivo pharmacokinetics and in vitro pharmacodynamics of nepafenac, amfenac, ketorolac, and bromfenac. J Cataract Refract Surg. 2007; 33:1539–1545.
Article31. Sasaki H, Yamamura K, Mukai T, Nishida K, Nakamura J, Nakashima M, et al. Pharmacokinetic prediction of the ocular absorption of an instilled drug with ophthalmic viscous vehicle. Biol Pharm Bull. 2000; 23:1352–1356.
Article32. Cha SH, Lee JS, Oum BS, Kim CD. Corneal epithelial cellular dysfunction from benzalkonium chloride (BAC) in vitro. Clin Experiment Ophthalmol. 2004; 32:180–184.
Article33. Epstein SP, Chen D, Asbell PA. Evaluation of biomarkers of inflammation in response to benzalkonium chloride on corneal and conjunctival epithelial cells. J Ocul Pharmacol Ther. 2009; 25:415–424.
Article34. Kusano M, Uematsu M, Kumagami T, Sasaki H, Kitaoka T. Evaluation of acute corneal barrier change induced by topically applied preservatives using corneal transepithelial electric resistance in vivo. Cornea. 2010; 29:80–85.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of 0.1% Bromfenac for Preventing Macular Edema after Cataract Surgery in Patients with Diabetes
- Effects of Perioperative Ketorolac Tromethamine on Intraocular Pressure in Glaucoma Patients Undergoing Cataract Surgery
- The Effect of Anti-inflammatory Agents on the Permeability of Trabecular Meshwork Cell Monolayers
- Effect of Topical Bromfenac as a Treatment of Cystoid Macular Edema Following Pars Plana Vitrectomy
- Current Guidelines for Non-Steroidal Anti-Inflammatory Drugs