Korean J Ophthalmol.
2020 Feb;34(1):46-55. 10.3341/kjo.2019.0044.
Effect of 0.1% Bromfenac for Preventing Macular Edema after Cataract Surgery in Patients with Diabetes
- Affiliations
-
- 1Department of Ophthalmology, Konyang University College of Medicine, Daejeon, Korea. astrix001@kyuh.ac.kr
- KMID: 2469286
- DOI: http://doi.org/10.3341/kjo.2019.0044
Abstract
- PURPOSE
To investigate the effect of 0.1% bromfenac sodium hydrate ophthalmic solution for prevention of macular edema after cataract surgery in patients with diabetes.
METHODS
A retrospective analysis of 75 patients with diabetes who underwent cataract surgery was performed. Thirty-eight patients (52 eyes) were instilled with 0.1% bromfenac solution (bromfenac group) and 37 patients (46 eyes) were not (control group).
RESULTS
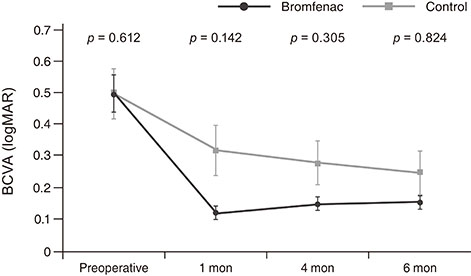
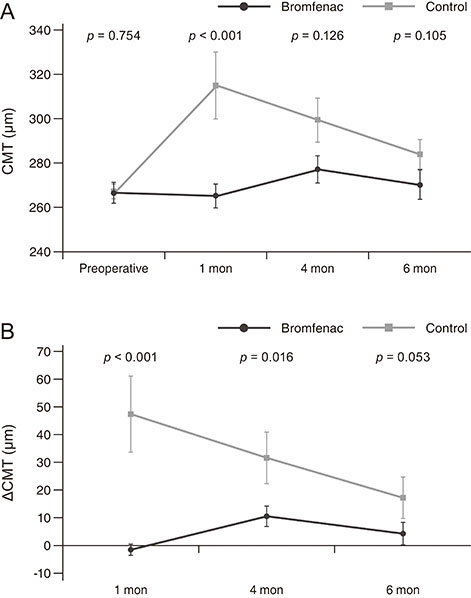
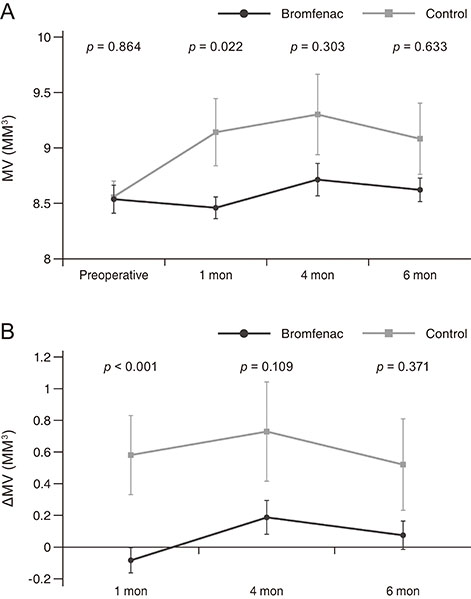
There were no significant preoperative between-group differences. Compared to the control group, at 1 month after surgery, the bromfenac group showed slightly better best-corrected visual acuity (0.12 ± 0.12 vs. 0.32 ± 0.42, p = 0.142), lower central macular thickness (265.58 ± 31.28 vs. 314.15 ± 76.11 µm, p < 0.001), and lower macular volume (8.46 ± 0.60 vs. 9.14 ± 1.53 mm³, p = 0.022). There were no significant differences between the two groups at 4 and 6 months postoperatively (p > 0.05). Mean changes in central macular thickness showed significant differences at 1 and 4 months postoperatively (−1.44 ± 11.72 and 10.44 ± 22.48 µm in bromfenac group vs. 47.19 ± 70.24 and 31.69 ± 48.04 µm in control group, p < 0.001 and p = 0.016) and mean changes in macular volume showed a significant difference at 1 month postoperatively (−0.08 ± 0.47 mm³ in bromfenac group vs. 0.58 ± 1.28 mm³ in control group, p < 0.001). There were no significant differences thereafter (p > 0.05).
CONCLUSIONS
Treatment with 0.1% bromfenac sodium hydrate ophthalmic solution showed good efficacy for preventing cystoid macular edema early after cataract surgery in patients with diabetes.
Keyword
MeSH Terms
Figure
Reference
-
1. Yonekawa Y, Kim IK. Pseudophakic cystoid macular edema. Curr Opin Ophthalmol. 2012; 23:26–32.
Article2. Kim SJ, Bressler NM. Optical coherence tomography and cataract surgery. Curr Opin Ophthalmol. 2009; 20:46–51.
Article3. Daien V, Papinaud L, Domerg C, et al. Incidence and characteristics of cystoid macular edema after cataract surgery. Ophthalmology. 2016; 123:663–664.
Article4. Rossetti L, Chaudhuri J, Dickersin K. Medical prophylaxis and treatment of cystoid macular edema after cataract surgery. The results of a meta-analysis. Ophthalmology. 1998; 105:397–405.5. Flach AJ. The incidence, pathogenesis and treatment of cystoid macular edema following cataract surgery. Trans Am Ophthalmol Soc. 1998; 96:557–634.6. Miyake K, Ibaraki N. Prostaglandins and cystoid macular edema. Surv Ophthalmol. 2002; 47:S203–S218.
Article7. Kim SJ, Flach AJ, Jampol LM. Nonsteroidal anti-inflammatory drugs in ophthalmology. Surv Ophthalmol. 2010; 55:108–133.8. Almeida DR, Johnson D, Hollands H, et al. Effect of prophylactic nonsteroidal antiinflammatory drugs on cystoid macular edema assessed using optical coherence tomography quantification of total macular volume after cataract surgery. J Cataract Refract Surg. 2008; 34:64–69.
Article9. Kim SJ, Equi R, Bressler NM. Analysis of macular edema after cataract surgery in patients with diabetes using optical coherence tomography. Ophthalmology. 2007; 114:881–889.
Article10. Brown RM, Roberts CW. Preoperative and postoperative use of nonsteroidal antiinflammatory drugs in cataract surgery. Insight. 1996; 21:13–16.11. Schmidl B, Mester U, Diestelhorst M, Konen W. Laser flare measurement with 3 different nonsteroidal anti-inflammatory drugs after phacoemulsification with posterior chamber lens implantation. Ophthalmologe. 1997; 94:33–37.12. Notivol R, Martinez M, Bergamini MV. Treatment of chronic nonbacterial conjunctivitis with a cyclo-oxygenase inhibitor or a corticosteroid. Pranoprofen Study Group. Am J Ophthalmol. 1994; 117:651–656.13. Kraff MC, Sanders DR, McGuigan L, Raanan MG. Inhibition of blood-aqueous humor barrier breakdown with diclofenac. A f luorophotometric study. Arch Ophthalmol. 1990; 108:380–383.14. Henderson BA, Gayton JL, Chandler SP, et al. Safety and efficacy of bromfenac ophthalmic solution (Bromday) dosed once daily for postoperative ocular inflammation and pain. Ophthalmology. 2011; 118:2120–2127.
Article15. Silverstein SM, Cable MG, Sadri E, et al. Once daily dosing of bromfenac ophthalmic solution 0.09% for postoperative ocular inflammation and pain. Curr Med Res Opin. 2011; 27:1693–1703.
Article16. Russo A, Costagliola C, Delcassi L, et al. Topical nonsteroidal anti-inflammatory drugs for macular edema. Mediators Inflamm. 2013; 2013:476525.
Article17. Schoenberger SD, Kim SJ. Nonsteroidal anti-inflammatory drugs for retinal disease. Int J Inflam. 2013; 2013:281981.
Article18. Koh CH, Chung SK. Effect of non-steroidal anti-inflammatory drugs on cystoid macular edema in diabetic patients after cataract surgery. J Korean Ophthalmol Soc. 2013; 54:427–431.
Article19. Chun BY, Kang SY, Song JS, Kim HM. Comparison of the effects of prophylactic nonsteroidal anti-inf lammatory drugs on macular edema after cataract surgery. J Korean Ophthalmol Soc. 2010; 51:935–940.20. Shimura M, Yasuda K. Topical bromfenac reduces the frequency of intravitreal bevacizumab in patients with branch retinal vein occlusion. Br J Ophthalmol. 2015; 99:215–219.
Article21. Flaxel C, Schain MB, Hamon SC, Francis PJ. Prospective randomized controlled trial of combination ranibizumab (Lucentis) and bromfenac (Xibrom) for neovascular age-related macular degeneration: a pilot study. Retina. 2012; 32:417–423.22. Gomi F, Sawa M, Tsujikawa M, Nishida K. Topical bromfenac as an adjunctive treatment with intravitreal ranibizumab for exudative age-related macular degeneration. Retina. 2012; 32:1804–1810.
Article23. Zweifel SA, Engelbert M, Khan S, Freund KB. Retrospective review of the efficacy of topical bromfenac (0.09%) as an adjunctive therapy for patients with neovascular age-related macular degeneration. Retina. 2009; 29:1527–1531.
Article24. Heier JS, Topping TM, Baumann W, et al. Ketorolac versus prednisolone versus combination therapy in the treatment of acute pseudophakic cystoid macular edema. Ophthalmology. 2000; 107:2034–2038.
Article25. Cho H, Wolf KJ, Wolf EJ. Management of ocular inflammation and pain following cataract surgery: focus on bromfenac ophthalmic solution. Clin Ophthalmol. 2009; 3:199–210.
Article26. Perry HD, Donnenfeld ED. An update on the use of ophthalmic ketorolac tromethamine 0.4%. Expert Opin Pharmacother. 2006; 7:99–107.
Article27. McGhee CN, Dean S, Danesh-Meyer H. Locally administered ocular corticosteroids: benefits and risks. Drug Saf. 2002; 25:33–55.28. Jones J, Francis P. Ophthalmic utility of topical bromfenac, a twice-daily nonsteroidal anti-inflammatory agent. Expert Opin Pharmacother. 2009; 10:2379–2385.
Article29. Wielders LH, Lambermont VA, Schouten JS, et al. Prevention of cystoid macular edema after cataract surgery in nondiabetic and diabetic patients: a systematic review and meta-analysis. Am J Ophthalmol. 2015; 160:968–981.
Article30. Shorstein NH, Liu L, Waxman MD, Herrinton LJ. Comparative effectiveness of three prophylactic strategies to prevent clinical macular edema after phacoemulsification surgery. Ophthalmology. 2015; 122:2450–2456.31. Kessel L, Tendal B, Jorgensen KJ, et al. Post-cataract prevention of inflammation and macular edema by steroid and nonsteroidal anti-inflammatory eye drops: a systematic review. Ophthalmology. 2014; 121:1915–1924.32. Jeong HK, Shin WB, Seo KY, Lee JH. Comparison of 1% prednisolone and 0.1% bromfenac solutions for preventing macular edema after cataract surgery. J Korean Ophthalmol Soc. 2016; 57:1834–1839.
Article33. Kato S, Fukada Y, Hori S, et al. Influence of phacoemulsification and intraocular lens implantation on the course of diabetic retinopathy. J Cataract Refract Surg. 1999; 25:788–793.
Article34. Baumal CR. Clinical applications of optical coherence tomography. Curr Opin Ophthalmol. 1999; 10:182–188.
Article35. Ahuja M, Dhake AS, Sharma SK, Majumdar DK. Topical ocular delivery of NSAIDs. AAPS J. 2008; 10:229–241.
Article36. Flach AJ. Topical nonsteroidal antiinflammatory drugs in ophthalmology. Int Ophthalmol Clin. 2002; 42:1–11.
Article37. Lindstrom R. The pharmacologic and pathophysiologic rationale for using NSAIDs in ocular inflammatory disease and ocular surgery. Int Ophthalmol Clin. 2006; 46:7–11.
Article38. Farah AE, Rosenberg F. Potential therapeutic applications of aspirin and other cyclo-oxygenase inhibitors. Br J Clin Pharmacol. 1980; 10:261S–278S.
Article39. Oka T, Shearer T, Azuma M. Involvement of cyclooxygenase-2 in rat models of conjunctivitis. Curr Eye Res. 2004; 29:27–34.
Article40. Sancilio LF, Nolan JC, Wagner LE, Ward JW. The analgesic and anti-inflammatory activity and pharmacologic properties of bromfenac. Arzneimittelforschung. 1987; 37:513–519.41. Waterbury LD, Silliman D, Jolas T. Comparison of cyclooxygenase inhibitory activity and ocular anti-inflammatory effects of ketorolac tromethamine and bromfenac sodium. Curr Med Res Opin. 2006; 22:1133–1140.42. Kida T, Kozai S, Takahashi H, et al. Pharmacokinetics and efficacy of topically applied nonsteroidal anti-inflammatory drugs in retinochoroidal tissues in rabbits. PLoS One. 2014; 9:e96481.
Article43. Baklayan GA, Patterson HM, Song CK, et al. 24-hour evaluation of the ocular distribution of (14)C-labeled bromfenac following topical instillation into the eyes of New Zealand White rabbits. J Ocul Pharmacol Ther. 2008; 24:392–398.
Article44. Endo N, Kato S, Haruyama K, et al. Efficacy of bromfenac sodium ophthalmic solution in preventing cystoid macular oedema after cataract surgery in patients with diabetes. Acta Ophthalmol. 2010; 88:896–900.
Article45. Kim SJ, Schoenberger SD, Thorne JE, et al. Topical nonsteroidal anti-inflammatory drugs and cataract surgery: a report by the American Academy of Ophthalmology. Ophthalmology. 2015; 122:2159–2168.46. Congdon NG, Schein OD, von Kulajta P, et al. Corneal complications associated with topical ophthalmic use of nonsteroidal antiinflammatory drugs. J Cataract Refract Surg. 2001; 27:622–631.
Article47. Lin JC, Rapuano CJ, Laibson PR, et al. Corneal melting associated with use of topical nonsteroidal anti-inflammatory drugs after ocular surgery. Arch Ophthalmol. 2000; 118:1129–1132.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of 1% Prednisolone and 0.1% Bromfenac Solutions for Preventing Macular Edema after Cataract Surgery
- Effect of Topical Bromfenac as a Treatment of Cystoid Macular Edema Following Pars Plana Vitrectomy
- The Effect of a 0.1% Bromfenac Solution on Diabetic Macular Edema
- Analysis of Postoperative Macular Edema in Cataract Patients with Diabetes using Optical Coherence Tomography
- Evaluation of Changes of Macular Thickness in Diabetic Retinopathy after Cataract Surgery