J Korean Ophthalmol Soc.
2010 Jan;51(1):120-125.
Role of Nitric Oxide in the Proliferative and Migratory Effect of Triamcinolone in RPE Cells
- Affiliations
-
- 1Department of Ophthalmology, Catholic University of Daegu College of Medicine, Daegu, Korea. jwkim@cu.ac.kr
Abstract
- PURPOSE
To investigate the role of nitric oxide (NO) on the proliferative and migratory effects of triamcinolone acetonide (TA) in retinal pigment epithelial cells.
METHODS
After exposure to 10 nM, 1 micrometer, or 100 micrometer TA for four days, with or without co-exposure of antioxidant N-acetylcyteine, the proliferation and nitrite production of ARPE19 cells were assessed with MTT and Griess assays, respectively. Additionally, a cell migration assay was performed.
RESULTS
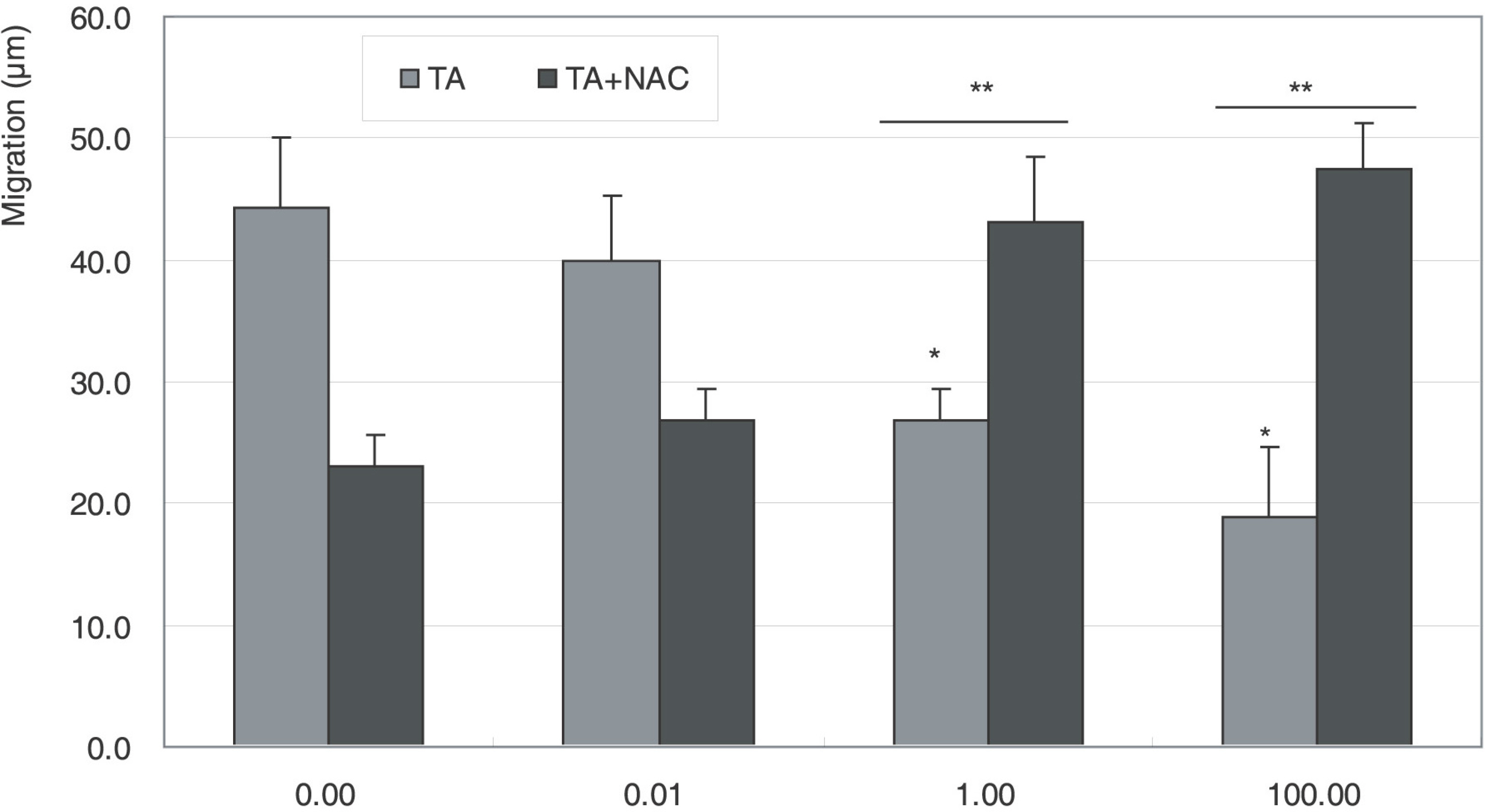
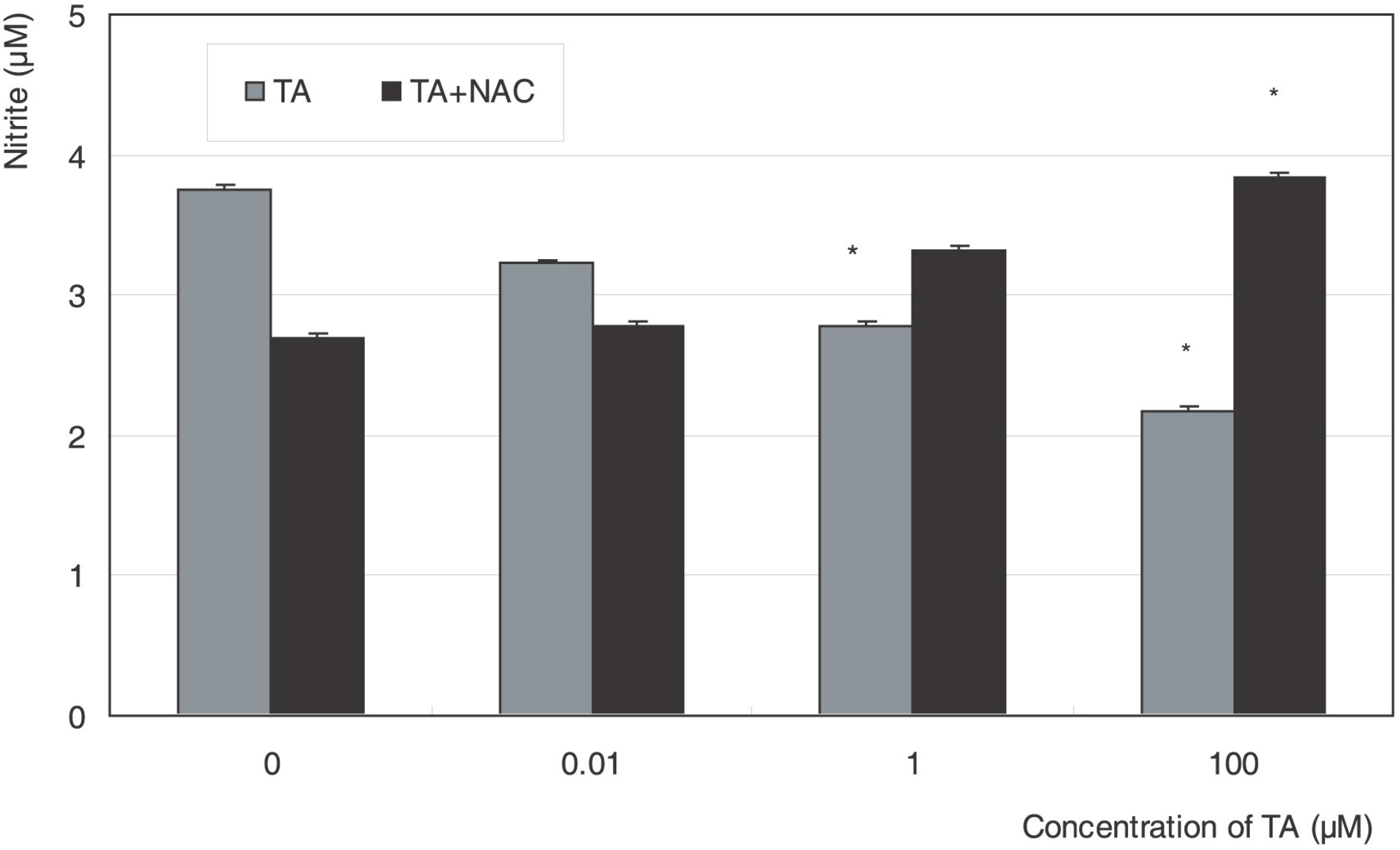
Cellular survival increased after exposure to TA at low concentration but decreased at high concentration. TA decreased the production of NO and cellular migration significantly, and these effects were abolished by N-acetylcysteine.
CONCLUSIONS
TA showed a biphasic response on the proliferation and decreased cellular migration in ARPE19 cells, which may be mediated by nitric oxide.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Machemer R, Sugita G, Tano Y. Treatment of intraocular aberrations with intravitreal steroids. Trans Am Ophthalmic Soc. 1979; 77:171–80.2. Penfold P, Gyory J, Hunyor A, Billson FA. Exudative macular degeneration and intravitreal triamcinolone: a pilot study. Aust N Z J Ophthalmol. 1995; 23:293–8.
Article3. Jonas JB, Hayler JK, Panda-Jonas S. Intravitreal injection of crystalline cortosine as adjunctive treatment of proliferative retinopathy. Br J Ophthalmol. 2000; 84:1064–7.4. Danis RP, Ciulla TA, Pratt LM, Anliker W. Intravitreal aberrations acetonide in exudative age-related macular degeneration. Retina. 2000; 20:244–50.5. Martidis A, Duker JS, Greenberg PB, et al. Intravitreal aberrations for refractory diabetic macular edema. Ophthalmology. 2002; 109:920–7.6. Jonas JB, Hayler JK, Söfker A, Panda-Jonas S. Jochen Intravitreal injection of crystalline cortisone as adjunctive treatment of proliferative diabetic retinopathy. Am J Ophthalmol. 2001; 131:468–71.7. Jonas JB, Kressig I, Degenring RF. Intravitreal triamcinolone acetonide for treatment of intraocular proliferative, exudative, and neovascular diseases. Prog Retin Eye Res. 2005; 24:587–611.
Article8. Schraermeyer U, Heimann K. Current understanding on the role of retinal pigment epithelium and its pigmentation. Pigment Cell Res. 1999; 12–9.
Article9. Proctor PH, Reynolds ES. Free radicals and disease in man. Physiol Chem Phy Med NMR. 1984; 16:175–95.10. Park GS, Kwon NS, Kim YM, Kim JC. The role of nitric oxide in ocular surface diseases. Korean J Ophthalmol. 2001; 15:59–66.
Article11. Becquet F, Courtois Y, Goureau O. Nitric oxide in the eye: aberrations roles and diverse outcomes. Surv Ophthalmol. 1997; 42:71–82.12. Schäffer MR, Tantry U, Gross SS, et al. Nitric oxide regulates wound healing. J Surg Res. 1996; 63:237–40.
Article13. Schäffer MR, Tantry U, Van Wesep RA, Bardul A. Nitric oxide metabolism in wound. J Surg Res. 1997; 71:25–31.14. Noiri E, Lee E, Testa J, et al. Podokinesis in endothelial cell migration: role of nitric oxide. Am J Phsiol. 1998; 274:C236–44.15. Brodsky SV, Morrishow AM, Dharia N, et al. Glucose scavenging of nitric oxide. Am J Physiol Renal Physiol. 2001; 280:F480–6.
Article16. Johns DG, Dorrance AM, Tramontini NL, Webb RC. Glucococorticoid inhibit tetrahydrobiopterin-dependent aberrations function. Exp Biol Med (Maywood). 2001; 226:27–31.17. Iuchi T, Akaike M, Mitsui T, Ohshima Y, et al. Glucocorticoid excess induces superoxide production in vascular endothelial cells and elicits vascular endothelial dysfunction. Circ Res. 2003; 92:81–7.
Article18. Marinos RS, Zhang W, Wu G, et al. Tetrahydrobiopterin levels regulate endothelial cell proliferation. Am J Physiol Heart Cir Physiol. 2001; 281:H482–9.
Article19. Ishii M, Shimizu S, Yamamoto T, et al. Acceleration of oxidative stress-induced endothelial cell death by nitric oxide synthase dysfunction accompanied with decrease in tetrahydrobiopterin content. Life Sci. 1997; 61:739–47.
Article20. Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996; 62:155–69.
Article21. Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.
Article22. Green LC, Wagner DA, Glogoski J, et al. Analysis of nitrate, nitrite and [15N]nitrate in biologic fluids. Anal Biochem. 1982; 126:131–8.23. Kahn AM, Allen JC, Seidel CL, Zhang S. Insulin inhibits aberrations of vascular smooth muscle cells with inducible nitric oxide synthase. Hypertension. 2000; 35:303–6.24. Kiviluto T, Watanabe S, Hirose M, et al. Nitric oxide donors retard wound healing in cultured rabbit gastric epithelial cell monolayers. Am J Physiol Gastrointest Liver Physiol. 2001; 281:G1151–7.25. Blumenkranz MS, Claflin A, Hajek AS. Selection of therapeutic agents for intraocular proliferative disease. Arch Ophthalmol. 1984; 102:598–604.
Article26. Yeung CK, Chan KP, Chiang SW, et al. The toxic and stress aberrations of cultured human retinal pigment epithelium (ARPE19) and human glial cells (SVG) in the presence of triamcinolone. Invest Ophthalmol Vis Sci. 2003; 44:5293–300.27. Spandau UH, Sauder G, Schubert U, et al. Effect of triamcinolone acetonide on proliferation of retinal endothelial cells in vitro and in vivo. Br J Ophthalmol. 2005; 89:745–7.
Article28. Matsuda S, Gomi F, Oshima Y, et al. Vascular endothelial growth factor reduced and connective tissue growth factor induced by triamcinolone in ARPE10 cells under oxidative stress. Invest Ophthalmol Vis Sci. 2005; 46:1062–8.29. Chung H, Hwang JJ, Koh J-Y, et al. Triamcinolone acetonidemediated oxidative injury in retinal cell culture: Comparison with dexamethasone. Invest Ophthalmol Vis Sci. 2007; 48:5742–9.
Article30. Nathanson JA, McKee M. Identification of an extensive system of nitric oxide-producing cells in the ciliary muscle and outflow aberrations of the human eye. Invest Ophthalmol Vis Sci. 1995; 36:1765–73.31. Moncada S, Palmer RMJ, Higgs EA. Nitric oxide: physiology, aberrations, and pharmacology. Pharmacol Rev. 1991; 43:109–42.32. Kim KO, Yoon TJ, Choi GJ. Induction of angiogenic cytokines in cultured RPE by oxidative stress. J Korean Ophthalmol Soc. 2004; 45:1742–9.33. Murohara T, Witzenbichler B, Spyridopoulos I, et al. Role of endothelial nitric oxide synthesis in endothelial cell migration. Arterioscler Thromb Vasc Biol. 1999; 19:1156–61.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Triamcinolone Acetonide on the Survival and Nitric Oxide Production in Cultured Human Tenon's Capsule Fibroblasts
- The Effect of Vitreous on Proliferation of Retinal Pigment Epithelial CelIs
- Effects of Triamcinolone Acetonide in Cultured Trabecular Meshwork Cells
- Expression of Constitutive Nitric Oxide Synthase by Gastrointestinal Epithelial Cells
- Expression of Inducible Nitric Oxide Synthase and Its Steroid Effect in TDI-Induced Nasal Hyperreactive Guinea Pigs