J Korean Ophthalmol Soc.
2010 Jan;51(1):112-119.
Ultrastructural Investigation of the Retinal Changes in Diabetic Rat (OLETF)
- Affiliations
-
- 1Department of Ophthalmology, School of Medicine, Yangsan Pusan National University, Yangsan, Korea.
- 2Department of Ophthalmology, School of Medicine, Pusan National University, Busan, Korea. bsoum@pusan.ac.kr
Abstract
- PURPOSE
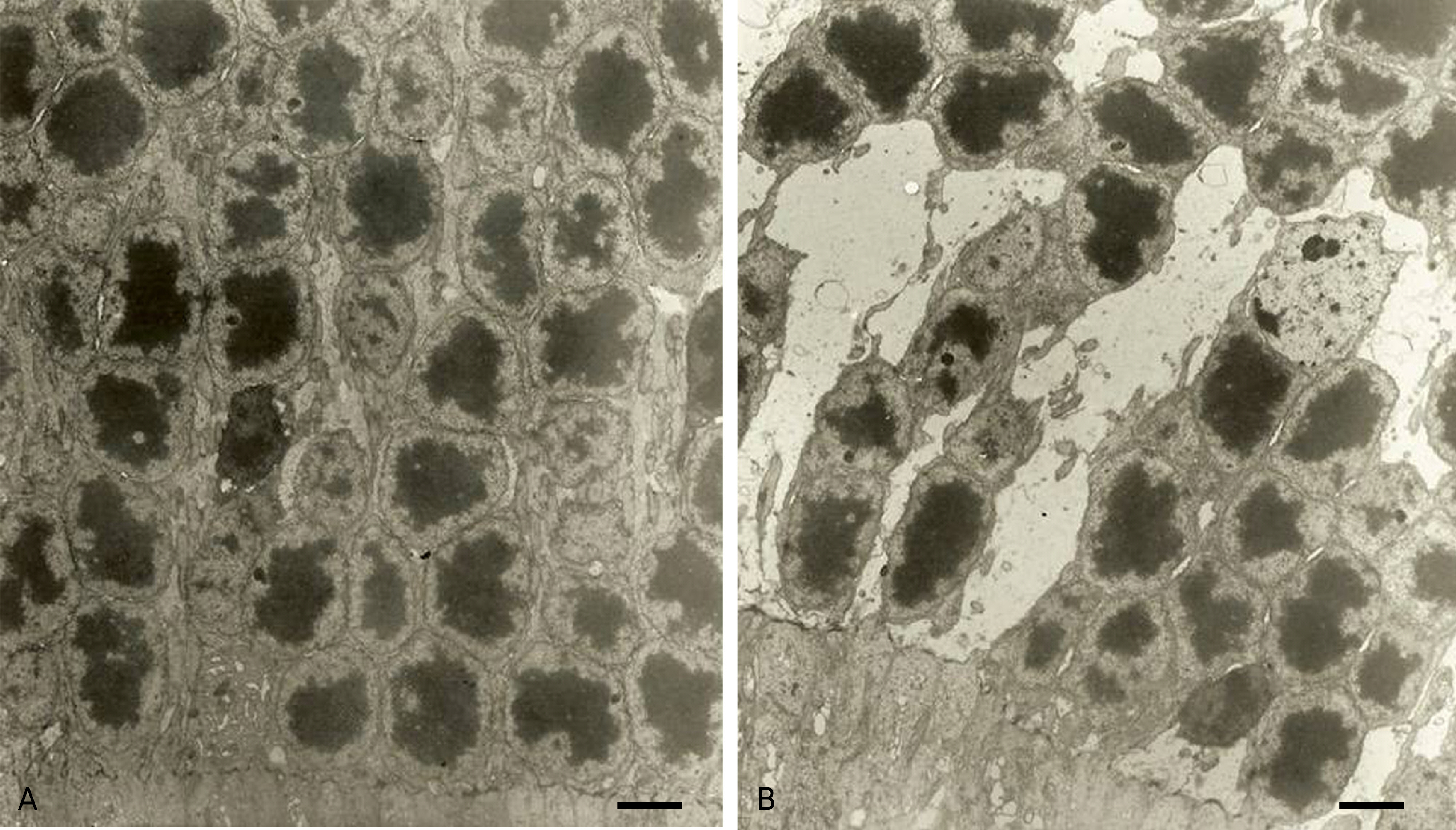
To compare retinal ultra-structures of diabetic rats (OLETF, Otsuka Long-Evans Tokushima Fatty) with those of agematched non-diabetic rats (LETO, Long-Evans Tokushima Otsuka) using transmission electron micrography (TEM).
METHODS
The body weights and blood sugar levels of the OLETF rats and LETO rats (n=5) were measured at 10 and 50 weeks of age. Using a TEM, we compared the ultra-structural changes between the retinas of the 50-week-old OLETF and LETO rats. We analyzed the sizes of the pericytes and the thicknesses of the retinal capillary basement membranes between the two groups. Comparisons were made using a Scion Image(R).
RESULTS
The mean body weight and blood sugar levels of the 50-week-old OLETF rats were significantly higher than those of the LETO rats (p(R)0.05). The thicknesses of the retinal capillary basement membranes in the outer plexiform layer and the size of pericytes were significantly increased in the OLETF rats at 50 weeks of age (p<0.05). The number of nuclei in the inner nuclear layer and the outer nuclear layer (photoreceptor cell nuclei) significantly decreased (p<0.05). However, the height of the RPE cells and basal in-foldings showed no significant differences between the OLETF and LETO rats.
CONCLUSIONS
The retinal changes in the OLETF rats were observed relatively early at 50 weeks of age. These changes are similar to those seen in human diabetic retinopathy. Change in the capillaries is one feature of early retinal change. OLETF rats may be a useful animal model in NIDDM to examine diabetic retinal changes.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Yoon YH. The mechanism of diabetic retinopathy, Retina. 2nd ed.Seoul: The Korean retina society;2008. p. 487–96.2. Gardiner TA, Stitt AW, Anderson HR, Archer DB. Selective loss of vascular smooth muscle cells in the retinal microcirculation of diabetic dogs. Br J Ophthalmol. 1994; 78:54–60.
Article3. Buchi ER, Kurosawa A, Tso MO. Retinopathy in diabetic hypertensive monkeys: a pathologic study. Graefes Arch Clin Exp Ophthalmol. 1996; 234:388–98.4. Kern TS, Engerman RL. A mouse model of diabetic retinopathy. Arch Ophthalmol. 1996; 114:986–90.
Article5. Kawano K, Hirashima T, Mori S, et al. Spontaneous long-term hyperglycemic rat with diabetic complications: Otsuka Long-Evans Tokishima Fatty (OLETF) strain. Diabetes. 1992; 41:1422–8.6. Stitt AW, Anderson HR, Gardiner TA, Archer DB. Diabetic retinopathy: quantitative variation in capillary basement membrane thickening in arterial or venous environments. Br J Ophthalmol. 1994; 78:133–7.
Article7. Anderson HR, Stitt AW, Gardiner TA, Archer DB. Diabetic retinopathy: morphometric analysis of basement membrane thickening of capillaries in different retinal layers within arterial and venous environments. Br J Ophthalmol. 1995; 79:1120–3.
Article8. Lee JS, Kim HK, Jung JH, Bae H. Analysis of corneal lesion using Scion Image®. J Korean Ophthalmol Soc. 2003; 44:1437–41.9. Cogan DG, Toussaint D, Kuwabara T. Retinal vascular patterns. IV. Diabetic retinopathy. Arch Ophthalmol. 1961; 66:366–78.10. Stitt AW, Gardiner TA, Archer DB. Histological and ultrastructural investigation of retinal microaneurysm development in diabetic patterns. Br J Ophthalmol. 1995; 79:362–7.11. Engerman RL, Kern TS. Hyperglycemia as a cause of diabetec retinopathy. Metabolism. 1986; 35:S20–3.12. Lu ZY, Bhutto IA, Amemiya T. Retinal changes in Otsuka long-evans Tokushima fatty rats (spontaneously diabetic rat)-possibility of a new experimental model for diabetic retinopathy. Jpn J Ophthalmol. 2003; 47:28–35.
Article13. Robison WG, Nagata M, Tillis TN, et al. Aldose reductase and pericyte-endothelial cell contacts in retina and optic nerve. Invest Ophthalmol Vis Sci. 1989; 30:2293–9.14. Cho HK, Lee DH. Ultrastructural changes of the retinal capillary basement membrane in the streptozotocin-induced diabetes rats. J Korean Ophthalmol Soc. 1995; 36:1473–80.15. Lee DC, Chang MH, Choi WS. An ultrastructural study on the early morphologic changes of the retina in the streptozotocin-induced diabetes rats. J Korean Ophthalmol Soc. 1999; 40:1884–92.16. Fisher RF. Comparison of the size of pericytes of the retinal capillaries in the normal and diabetic state. Trans Ophthalmol Soc U K. 1980; 100:90–5.17. Miyamura N, Bhutto IA, Amemiya T. Retinal capillary changes in Otsuka Long-Evans Tokushima fatty rats (spontaneous diabetic strain). Electronmicroscopic study. Ophthalmic Res. 1999; 31:358–66.18. Matsuura T, Yamagishi S, Kodama Y, et al. Otsuka Long-Evans Tokushima fatty (OLETF) rat is not a suitable animal model for the study of angiopathic diabetic retinopathy. Int J Tissue React. 2005; 27:59–62.19. Yonemura D, Aoki T, Tsuzuki K. Electroretinogram in diabetic retinopathy. Arch Ophthalmol. 1962; 68:19–24.
Article20. Amemiya T. Dark adaptation in diabetes. Ophthalmologica. 1977; 174:322–6.21. Shirao Y, Segawa Y, Higashide T, et al. Biochemical and electrophysiological alterations in the OLETF rat retina. Shima K, editor. Obesty and NIDDM: Lessons from the OLETF rat. Amsterdam: Elsevier Science B.V.;1999. 3:chap.p. 15.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ultrastructural Changes in Corneas of Diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) Rats
- Ultrastructural Changes of The Retinal Capillary Basement Membrane in The Streptozotocin-Induced Diabetic Rats
- An Ultrastructural Study on the Early Morphologic Changes of the Retina in Streptozotocin-induced Diabetic Rats
- Gene Microarray Related with Apoptosis in Diabetic OLETF Keratocytes
- Involvement of VEGF in both Cell Apoptosis and Survival in the Retina of Type 2 Diabetic OLETF Rats