J Korean Diabetes Assoc.
2007 Sep;31(5):391-401.
Therapeutic Efficacy of Combined Therapy with Once Daily Insulin Glargine and Once Daily Glimepiride in Korean Type 2 Diabetic Patients
- Affiliations
-
- 1Department of Internal Medicine, Eulji University School of Medicine.
Abstract
-
BACKGROUND: Once daily injection and 24 hour lasting glucose lowering effect of insulin glargine had recently changed a perception about the early insulin treatment of type 2 diabetic patients. This study was performed to investigate therapeutic efficacy of combined therapy with insulin glargine and glimepiride in Korean type 2 diabetic patients, who had received oral hypoglycemic agents (OHA) or conventional insulin therapy.
METHODS
Total of 192 patients who needed to change the previous therapy because of uncontrolled diabetes or hypoglycemia were included and followed for about 6 months. Two groups of prior treatment modality were analyzed; OHA group (n = 54, 28.1%), conventional insulin therapy group in combination with or without OHA group (n = 138, 71.9%). The primary end point was changes in HbA1c according to baseline characteristics such as prior treatment modality, HbA1C, c-peptide, duration of diabetes mellitus, body mass index and prior used conventional insulin doses. Secondary end point was the dose conversion ratio of insulin glargine to prior used insulin in patients who had one or two insulin therapy. We also evaluated the level of the patients' satisfaction on the glucose lowering effects and the convenience for use of device.
RESULTS
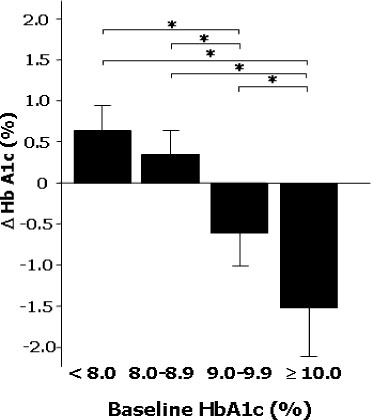
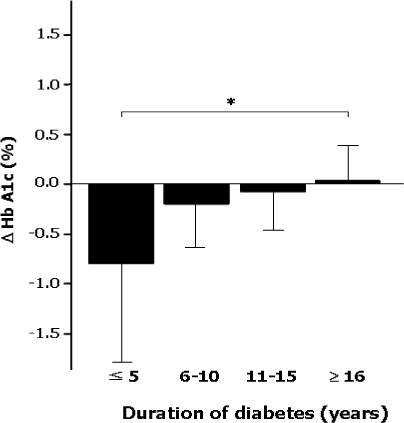
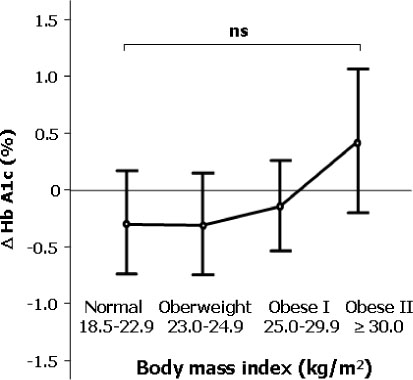
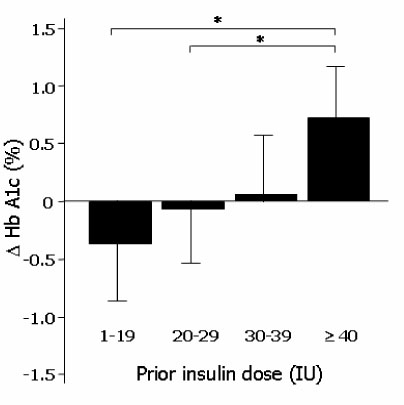
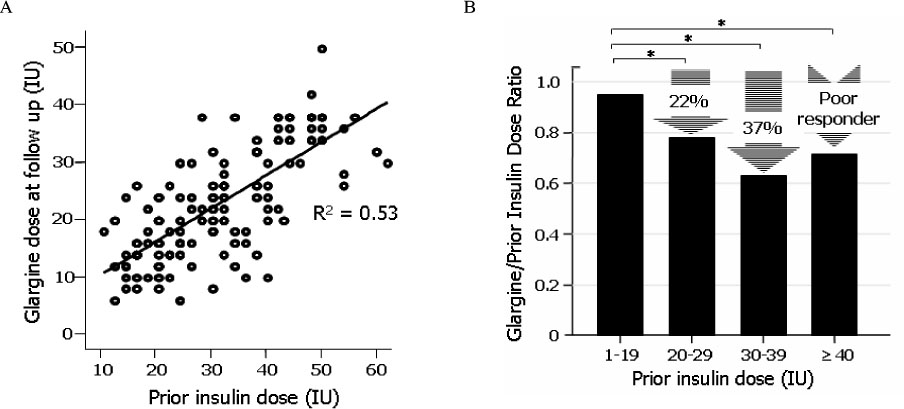
The differences of HbA1c according to prior treatment groups were -0.78 +/- 1.76 % in OHA group and 0.07 +/- 1.44 % in conventional insulin group with or without OHA group. The HbA1c improved better when baseline HbA1c was higher than 9%, c-peptide was higher than 0.6 ng/mL, duration of diabetes was shorter than 15 years, BMI was lower than 30 kg/m2 and prior conventional insulin dose was less than 30 IU. However, those effects were attenuated in subjects having duration of diabetes longer than 16 years, BMI higher than 30 kg/m2 and prior insulin dose more than 40 IU. Dose conversion ratio of the insulin glargine to prior insulin was 0.78 +/- 0.30 and showed a tendency to increase in patients who have prior insulin dose more than 40 IU. The scores of the patients' subjective satisfaction on insulin glargine were all high, irrespective of the changes of HbA1c.
CONCLUSIONS
Once daily injection of insulin glargine and oral ingestion of glimepiride can be recommended as one of starting insulin regimen for patients who are not adequately controlled by OHA alone or as once daily regimen for whom treated with one or two conventional insulin therapy.
MeSH Terms
Figure
Reference
-
2. The Diabetes Control and Complications Trial (DCCT) Research Group. Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. The Am J Cardiol. 1995. 75:894–903.3. Bonora E. Postprandial peaks as a risk factor for cardiovascular disease. Int J Clin Pract Suppl. 2002. 129:5–11.4. Ceriello A. Postprandial Hyperglycemia and Diabetes Complications: Is It Time to Treat? Diabetes. 2005. 54:1–7.5. Stratton JM, Alder AJ, Neil HAW, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. The UK Prospective Diabetes Study Group: Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000. 321:405–412.6. Bonora E, Muggeo M. Postprandial blood glucose as a risk factor for cardiovascular disease in Type II diabetes: the epidemiological evidence. Diabetologia. 2001. 44:2107–2114.7. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes UKPDS 33. Lancet. 1998. 352:837–853.8. American Diabetes Association. Clinical practice recommandation 2004; Standards medical care in diabetes. Diabetes Care. 2004. 27:Suppl 1. S15–S35.9. Wright A, Burden AC, Paisey RB, Cull CA, Holman RR. U.K. Prospective Diabetes Study Group: Sulfonylurea inadequacy: efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the U.K. Prospective Diabetes Study (UKPDS 57). Diabetes Care. 2002. 25(2):330–336.10. The Diabetes Control and Complication Trial (DCCT) Research Group. Effect of intensive treatment of diabetes on the development and progression of long-term complication in insulin-dependent diabetes mellitus. N Eng J Med. 1993. 329:977–986.11. Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, Kojima Y, Furuyoshi N, Shichiri M. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non insulin dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995. 28:103–117.12. Shichiri M, Kishikawa H, Ohkubo Y, Wake N. Long-term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care. 2000. 23:Suppl 2. B21–B29.13. Wake N, Hisashige A, Katayama T, Kishikawa H, Ohkubo Y, Sakai M, Araki E, Shichiri M. Cost-effectiveness of intensive insulin therapy for type 2 diabetes: a 10-year follow-up of the Kumamoto study. Diabetes Res Clin Pract. 2000. 48:201–210.17. Fritsche A, Schweitzer MA, Haring HU. Glimepiride combined with morning insulin glargine, bedtime neutral protamine hagedorn insulin, or bedtime insulin glargine in patients with type 2 diabetes. A randomized, controlled trial. Ann Intern Med. 17. 138(12):952–959.18. Wang F, Carabino JM, Vergara CM. Insulin glargine: a systematic review of a long-acting insulin analogue. Clin Ther. 2004. 26(7):1179–1183.19. McKeage K, Goa KL. Insulin glargine: a review of its therapeutic use as a long-acting agent for the management of type 1 and 2 diabetes mellitus. Drugs. 200. 61(11):1599–1624.20. Rosenstock J, Dailey G, Massi-Benedetti M, Fritsche A, Lin Z, Salzman A. Reduced hypoglycemia risk with insulin glargine: a meta-analysis comparing insulin glargine with human NPH insulin in type 2 diabetes. Diabetes Care. 2005. 28:950–955.21. Yki-Jarvinen H, Dressler A, Ziemen M. HOE 901/00s Study Group. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. HOE 901/3002 Study Group. Diabetes Care. 2000. 23:1130–1136.22. Rosenstock J, Schwartz SL, Clark CM Jr, Park GD, Donley DW, Edwards MB. Basal insulin therapy in type 2 diabetes. Diabetes Care. 2001. 24:631–636.23. Wang XL, Lu JM, Pan CY, Mu YM, Dou JT, Ba JM, Wang X. Evaluation of the superiority of insulin glargine as basal insulin replacement by continuous glucose monitoring system. Diabetes Res Clin Pract. 2007. 76:30–36.24. Yokoyama H, Tada J, Kamikawa F, Kanno S, Yokota Y, Kuramitsu M. Efficacy of conversion from bedtime NPH insulin to morning insulin glargine in type 2 diabetic patients on basal-prandial insulin therapy. Diabetes Res Clin Pract. 2006. 73:35–40.25. Pugh JA, Wagner ML, Sawyer J, Ramirez G, Tuley M, Freidberg SJ. Is combination sulfonylurea and insulin therapy useful in NIDDM patients? A meta-analysis. Diabetes Care. 1992. 15:953.26. Rosenstock J, Sugimoto D, Strange P, Stewart JA, Soltes-Rak E, Dailey G. Triple therapy in type 2 diabetes: insulin glargine or rosiglitazone added to combination therapy of sulfonylurea plus metformin in insulin-naive patients. Diabetes Care. 2006. 29:554–559.27. Johnson JL, Wolf SL, Kabadi UM. Efficacy of insulin and sulfonylurea combination therapy in type II diabetes: a meta-analysis of the randomized placebo-controlled trials. Arch Intern Med. 1996. 156:259.28. Riddle MC, Rosenstock J, Gerich J. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003. 26:3080–3086.29. Eliaschewitz FG, Calvo C, Valbuena H, Ruiz M, Aschner P, Villena J, Ramirez LA, Jimenez J. Therapy in type 2 diabetes: insulin glargine vs. NPH insulin both in combination with glimepiride. Arch Med Res. 2006. 37:495–501.30. Pan CY, Sinnassamy P, Chung KD, Kim KW. Insulin glargine versus NPH insulin therapy in Asian Type 2 diabetes patients. Diabetes Res Clin Pract. 2007. 3687:1–8.31. Yki-Jarvinen H, Kauppinen-Makelin R, Tiikkainen M, Vahatalo M, Virtamo H, Nikkila K, Tulokas T, Hulme S, Hardy K, McNulty S, Hanninen J, Levanen H, Lahdenpera S, Lehtonen R, Ryysy L. Insulin glargine or NPH combined with metformin in type 2 diabetes: the LANMET study. Diabetologia. 2006. 49:442–451.32. Wulffele MG, Kooy A, Lehert P, Bets D, Ogterop JC, Borger van der Burg B, Donker AJ, Stehouwer CD. Combination of insulin and metformin in the treatment of type 2 diabetes. Diabetes Care. 2002. 25:2133–2140.33. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic ROC curve. Radiology. 1982. 143:29–36.36. The Asia-Pacific perspective. Redefining obesity and its treatment. 2000. Available from http://www.diabetes.com.au/downloads.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Short-term Therapeutic Efficacy of Different Oral Hypoglycemic Agents Combined with Once Daily Insulin Glargine in Type 2 Diabetic Subjects with Failure of Sulfonylurea and Metformin Combination
- Effect of Insulin Glargine in Adolescents with Uncontrolled type 1 Diabetes Mellitus
- Exenatide versus Insulin Lispro Added to Basal Insulin in a Subgroup of Korean Patients with Type 2 Diabetes Mellitus
- Determining the Factors that Influence the Insulin Requirements in Type 2 Diabetic Patients
- Efficacy and Safety of Glimepiride: A Novel Sulfonylurea Drug compared with Gliclazide in the Treatment of Type 2 Diabetes Mellitus: an Open , Randomized Comparative Multi - Center Clinical Study