J Korean Diabetes Assoc.
2007 Jul;31(4):336-342. 10.4093/jkda.2007.31.4.336.
Short-term Therapeutic Efficacy of Different Oral Hypoglycemic Agents Combined with Once Daily Insulin Glargine in Type 2 Diabetic Subjects with Failure of Sulfonylurea and Metformin Combination
- Affiliations
-
- 1Department of Internal Medicine, Kwandong University College of Medicine.
- 2Department of Preventive Medicine and Public Health, Cheju National University College of Medicine.
- KMID: 2222521
- DOI: http://doi.org/10.4093/jkda.2007.31.4.336
Abstract
-
Backgroud: Although the extended duration of action of insulin glargine supports a convenient once daily injection, the combination with other short acting insulins or oral hypoglycemic agents is required to control postprandial hyperglycemia in type 2 diabetes. The present study was designed to compare the short-term therapeutic efficacy of oral hypoglycemic agents with once daily insulin glargine, switching from a multiple daily injection regimen.
METHODS
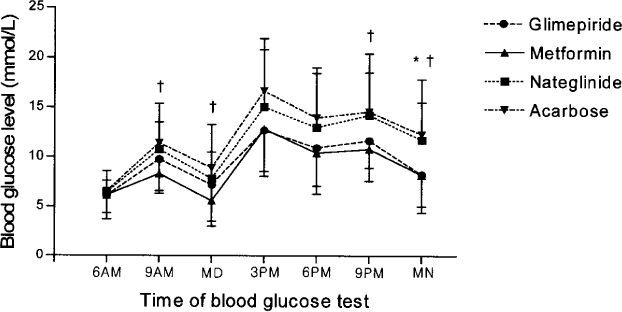
After control with the intensive regimen (daily lispro insulin and glargine) during 5~7 days, 80 in-patients with type 2 diabetes were randomized and treated with four oral hypoglycemic agents (glimepiride 4 mg qd, metformin 500 mg bid, nateglinide 90 mg tid, or acarbose 100 mg tid) plus once daily insulin glargine during 5 days. Blood glucose concentration was recorded by seven daily estimations (before each meal, 2 hours after each meal, and bedtime). Blood glucose concentrations and area under the curves (AUCs) of blood glucose were compared among four groups.
RESULTS
The area under the curve of blood glucose of metformin, glimepiride, nateglinide, and acarbose groups were 165.5 +/- 46.0, 178.5 +/- 36.5, 209.9 +/- 55.1, and 224.9 +/- 55.8 mmol/L/hr respectively. Blood glucose concentrations and area under the curves of blood glucose of glimepiride and metformin groups were significantly lower than those of acarbose group. Also, those of metformin group were significantly lower than those of nateglinide group.
Conclusions
Metformin or glimepiride are more effective oral hypoglycemic agent than nateglinide or acarbose in the combination with insulin glargine in type 2 diabetic subjects with failure of sulfonylurea and metformin combination.
MeSH Terms
Figure
Reference
-
1. U.K. prospective diabetes study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998. 352:837–853.2. Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000. 321:405–412.3. U.K. prospective diabetes study Group. Overview of 6 years' therapy of type II diabetes: a progressive disease. Diabetes. 1995. 44:1249–1258.5. Yki-Jarvinen H, Dressler A, Ziemen M. HOE 901/3002 Study Group. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. Diabetes Care. 2000. 23:1130–1136.6. Gillies PS, Figgitt DP, Lamb HM. Insulin glargine. Drugs. 2000. 59:253–260.7. Bolli GB, Di Marchi RD, Park GD, Pramming S, Koivisto VA. Insulin analogues and their potential in the management of diabetes mellitus. Diabetologia. 1999. 42:1151–1167.8. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004. 27:S5–S10.10. Haffner SM, Mettinen H, Stern MP. The homeostasis model in the San Antonio Heart Study. Diabetes Care. 1997. 20:1087–1092.11. Matthews DR, Hosker JP, Rudenski AS. Homeostasis model assessment: insulin resistance and beta-cell function from plasma fasting glucose and insulin concertrations in man. Diabetologia. 1985. 28:412–419.12. Janka HU, Plewe G, Riddle MC, Kliebe-Frisch C, Schweitzer MS, Yki-Jarvinen H. Comparison of basal insulin added to oral agents versus twice-daily premixed insulin as initial insulin therapy for type 2 diabetes. Diabetes Care. 2005. 28:254–259.14. Defronzo RA, Barzilai N, Simonson DC. Mechanism of metformin action in obese and lean non-insulin dependent diabetic subjects. J Clin Endocrinol Metab. 1991. 73:1294–1301.15. Klip A, Leiter LA. Cellular mechanism of action of metformin. Diabetes Care. 1990. 13:696–704.17. Horton ES, Clinkingbeard C, Gatlin M, Foley J, Mallows S, Shen S. Nateglinide alone and in combination with metformin improves glycemic control by reducing mealtime glucose levels in type 2 diabetes. Diabetes Care. 2000. 23:1660–1665.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Therapeutic Efficacy of Combined Therapy with Once Daily Insulin Glargine and Once Daily Glimepiride in Korean Type 2 Diabetic Patients
- Combination Therapy of Oral Hypoglycemic Agents in Patients with Type 2 Diabetes Mellitus
- A Pregnant Woman with Type 2 Diabetes Unintentionally Exposed to Metformin and Voglibose until the Second Trimester of Pregnancy: A Case Report
- Antihyperglycemic agent combination therapy for patients with type 2 diabetes mellitus
- DPP-4 Inhibitors as a New Option for the Management of Type 2 Diabetes