J Korean Assoc Oral Maxillofac Surg.
2010 Oct;36(5):392-401.
Effect of adipose-derived stem cells on bone healing on titanium implant in tibia of diabetes mellitus induced rats
- Affiliations
-
- 1Department of Oral and Maxillofacial Surgery, Collage of Medicine, Dona-A Universtiy, Busan, Korea.
- 2Department of Oral and Maxillofacial Surgery, School of Dentistry, Pusan National University, Yangsan, Korea. kuksjs@pusan.ac.kr
Abstract
- INTRODUCTION
Diabetes mellitus, as a major health problem for the elderly has been shown to alter the properties of the bone and impair bone healing around a titanium implant in both humans and animals. The aim of this study was to examine the effect of adipose-derived stem cells on the healing process around a titanium implant in streptozotocin-induced diabetic rats.
MATERIALS AND METHODS
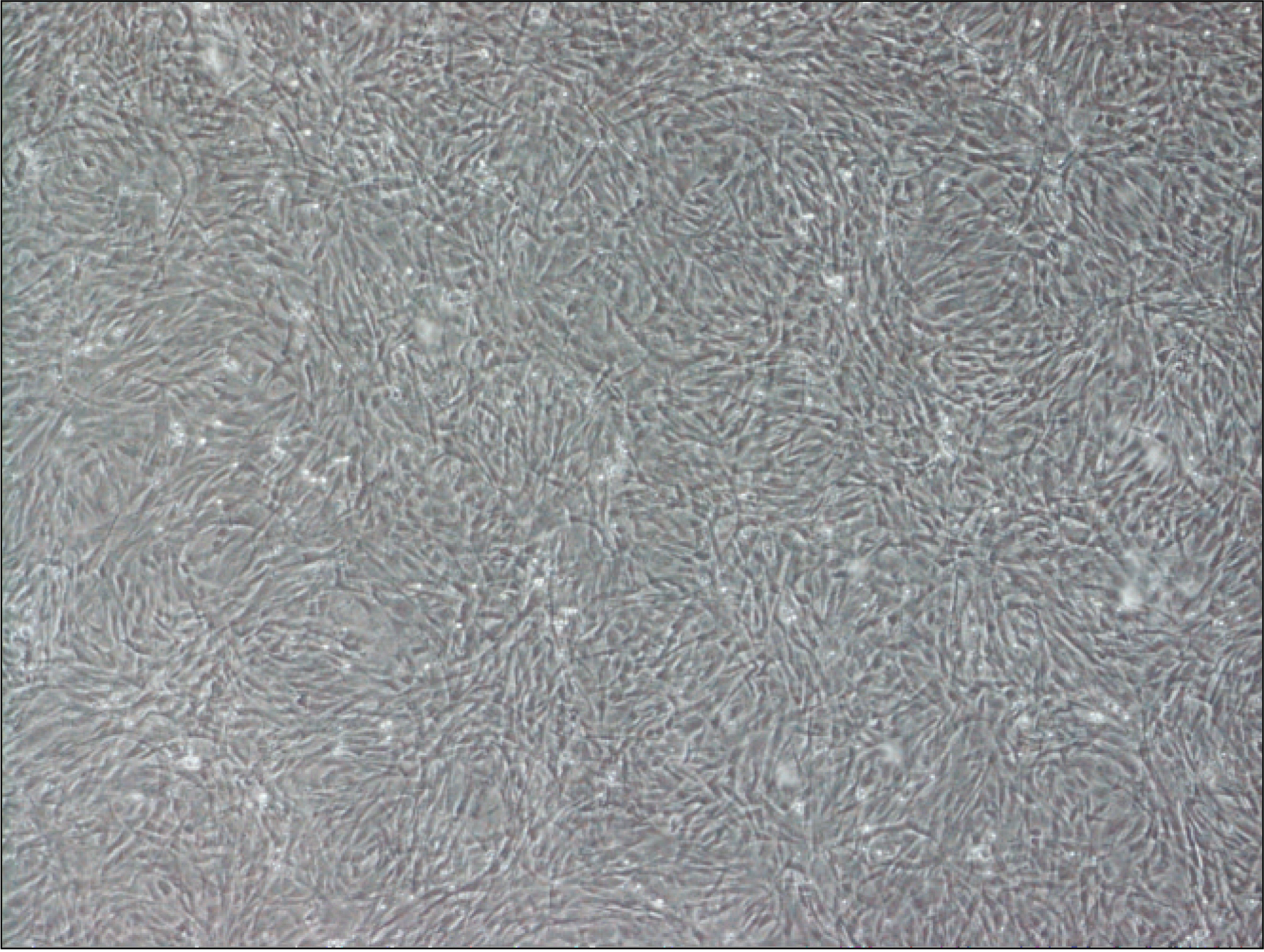
Thirteen rats were divided into two groups: adipose-derived stem cells injected group and a control group. A titanium screw implant (diameter: 2.0 mm, length: 3.5 mm) was placed into both tibia of 13 rats: 13 right tibia as the control group and 13 left tibia as the experimental group. The rats were sacrificed at different intervals (1, 2, and 4 weeks) after implantation for histopathology observations and immunohistochemistric analysis.
RESULTS
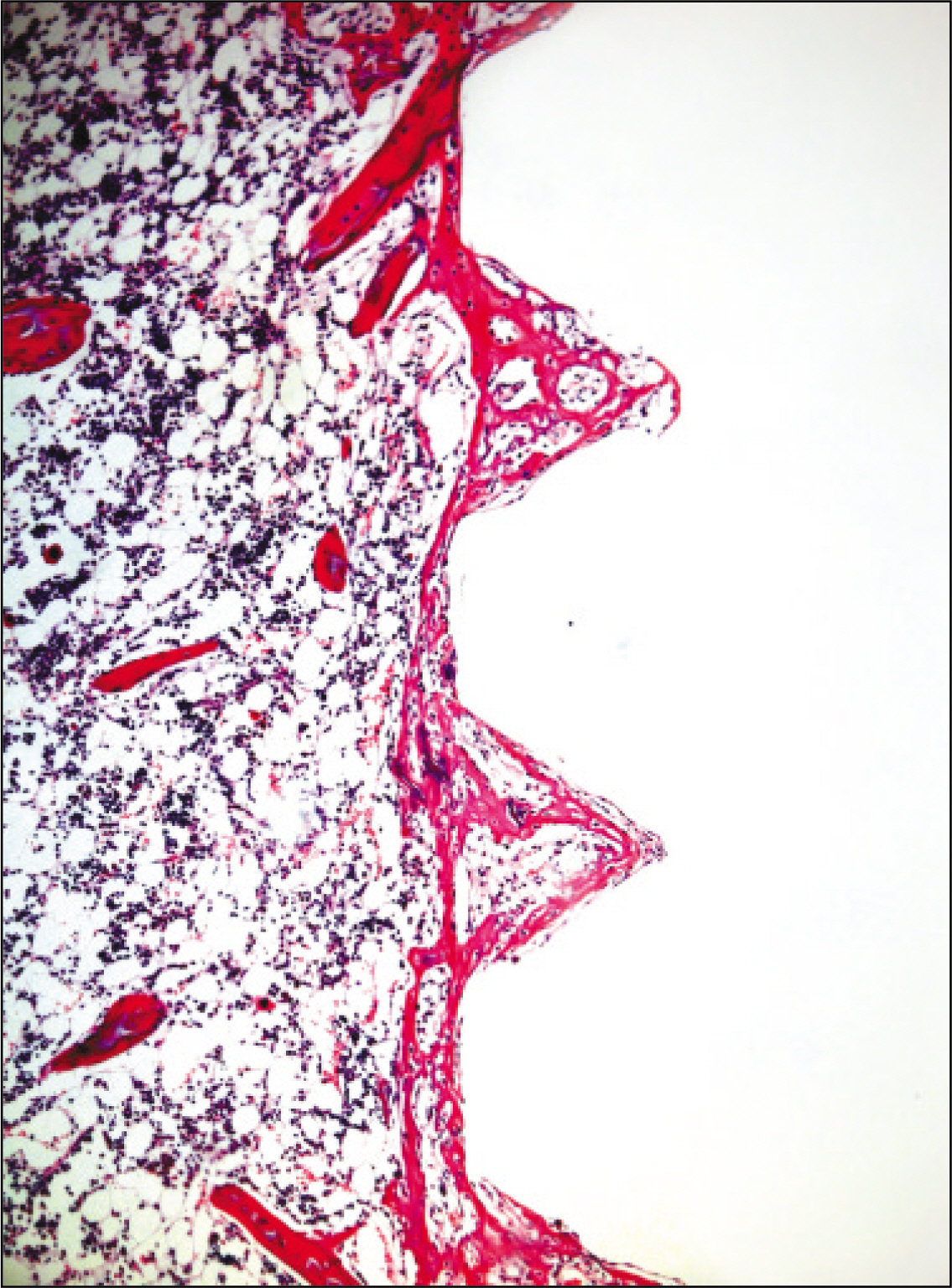
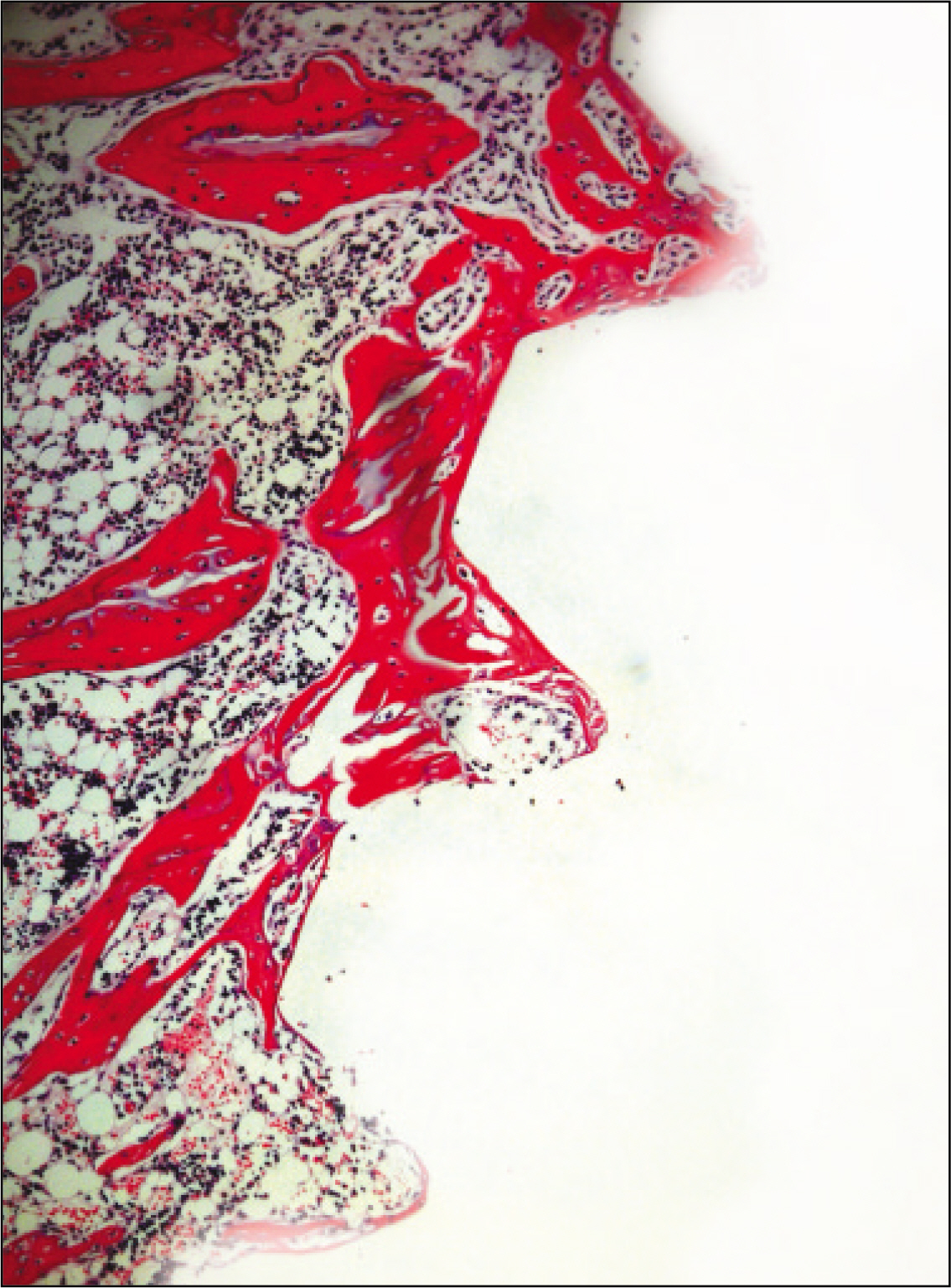
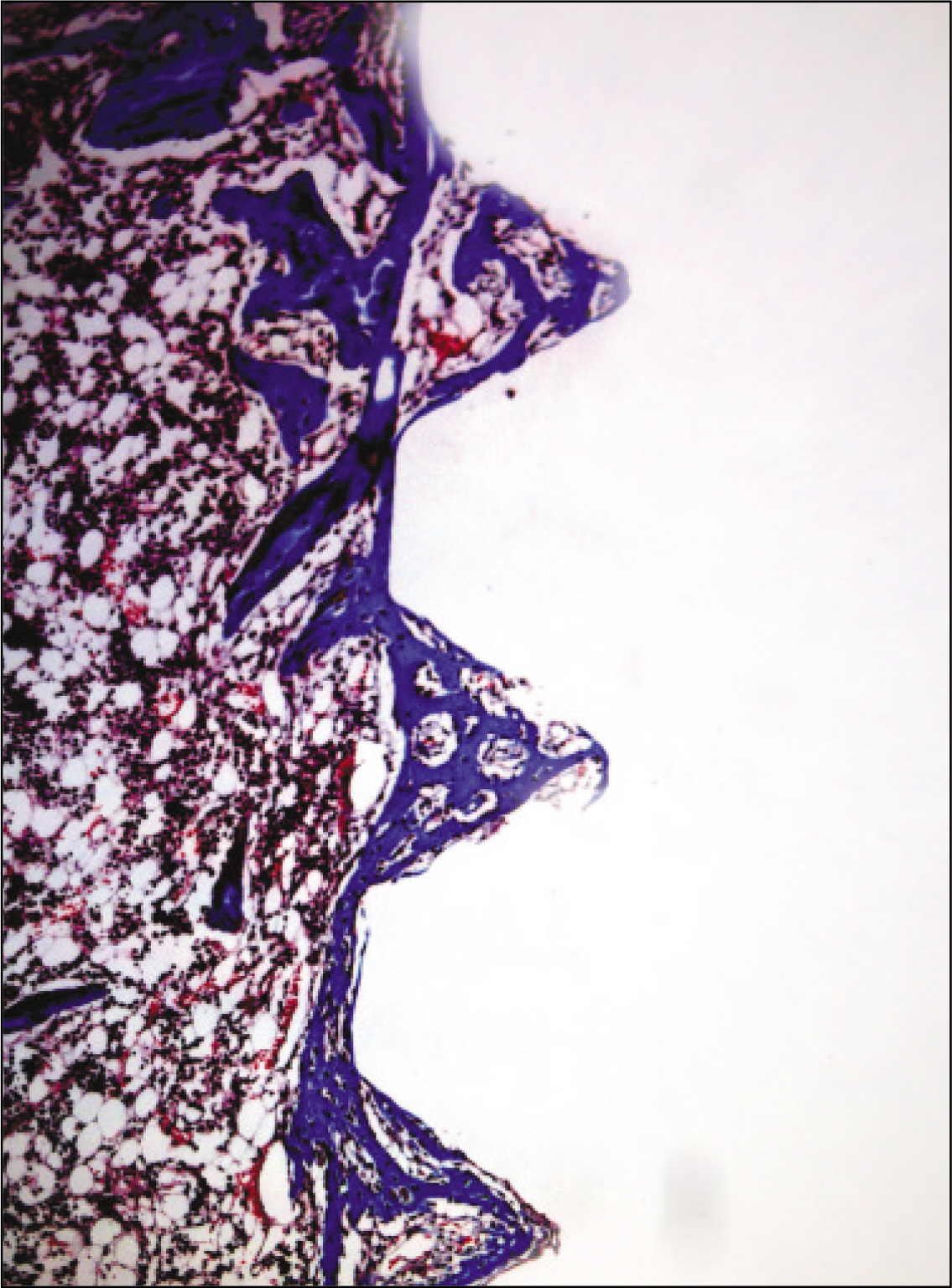
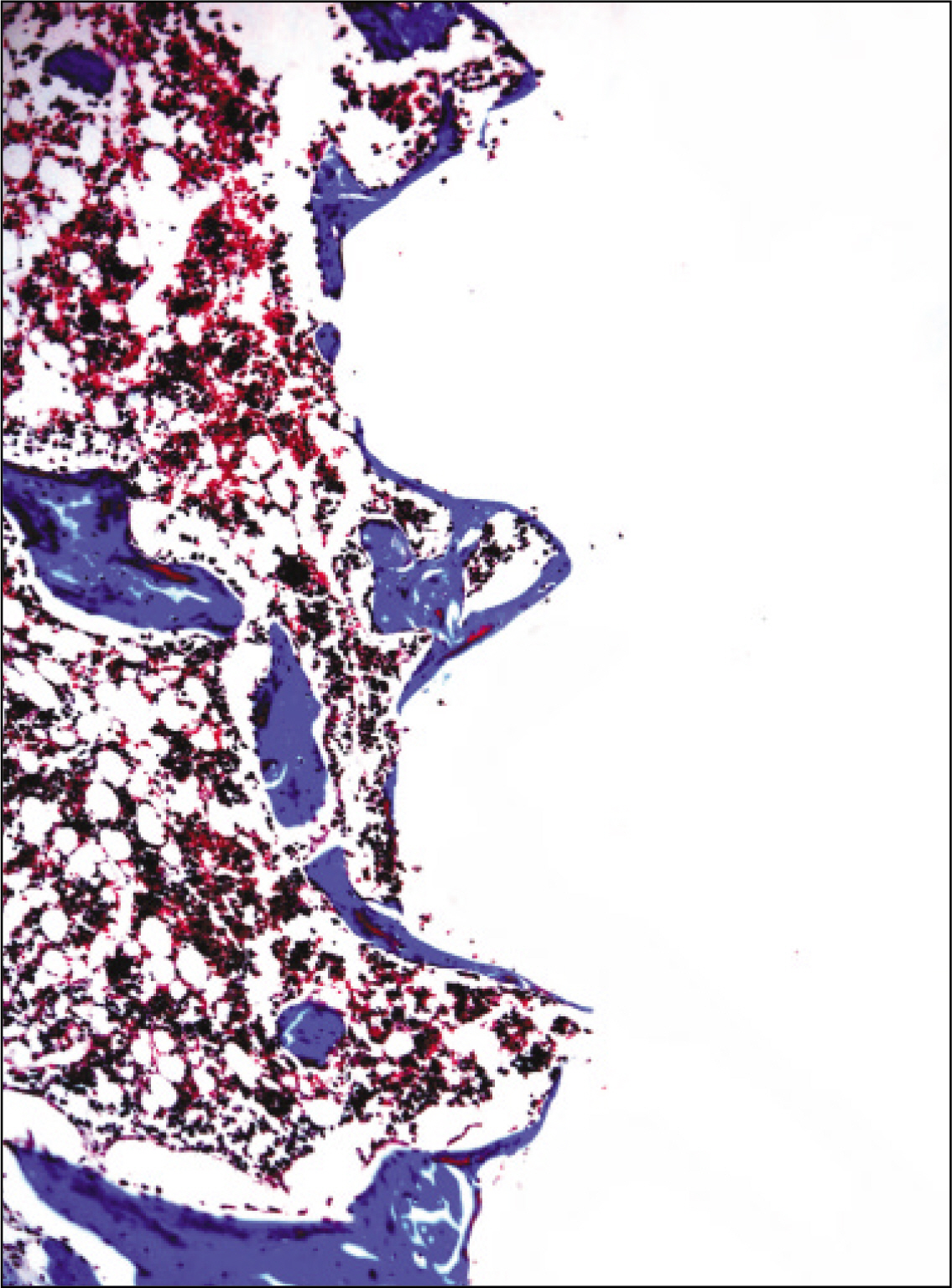
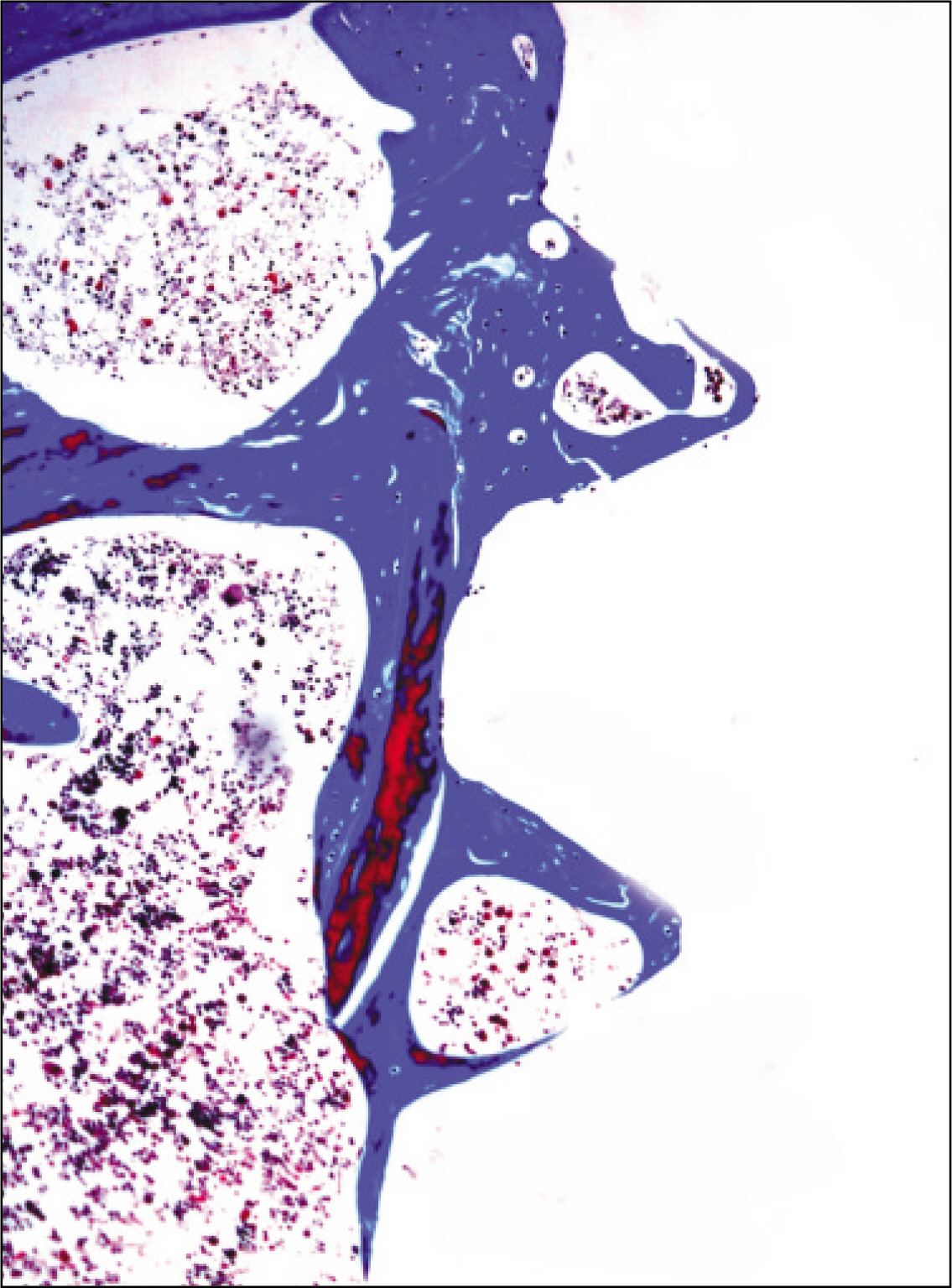
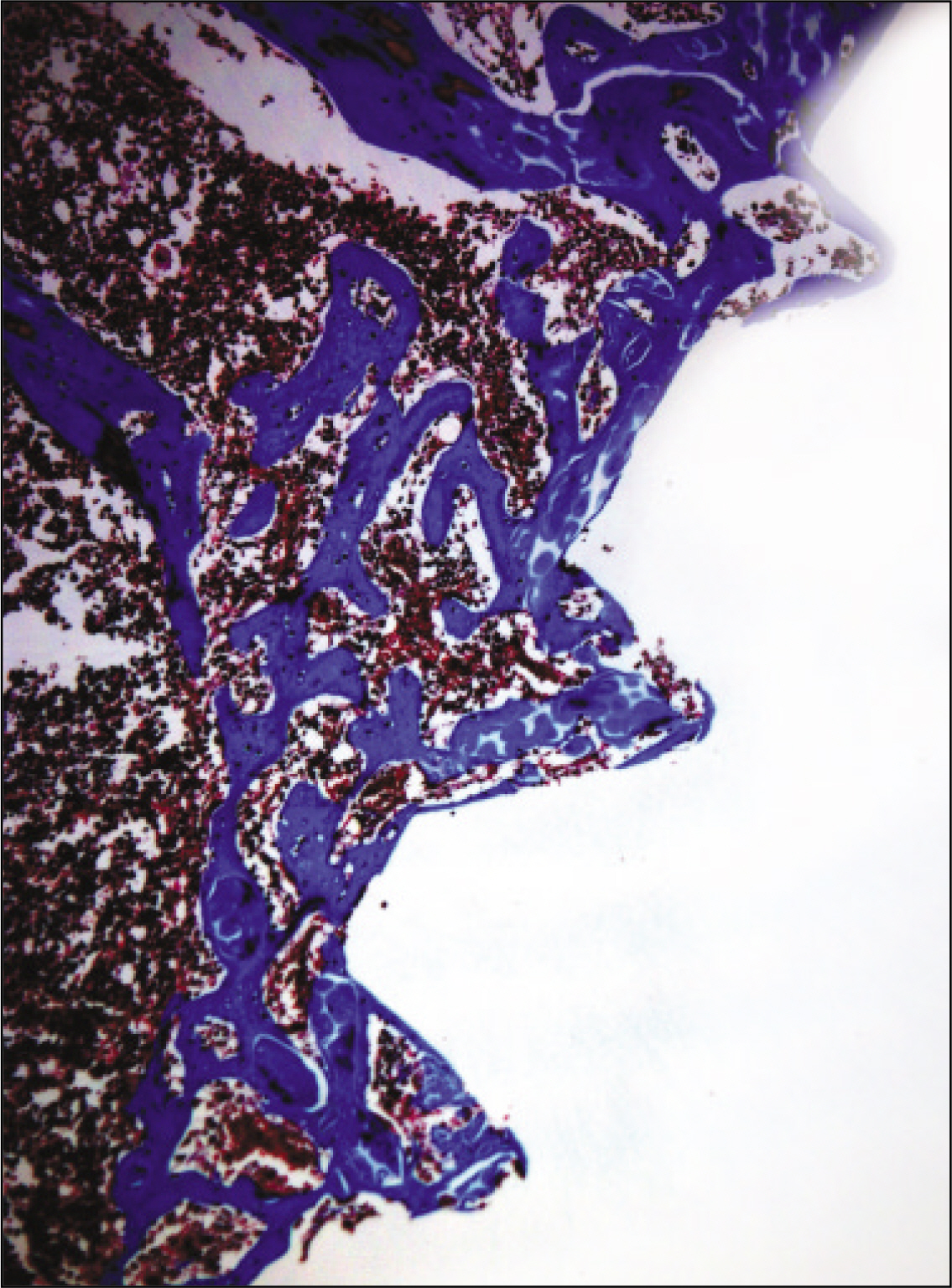
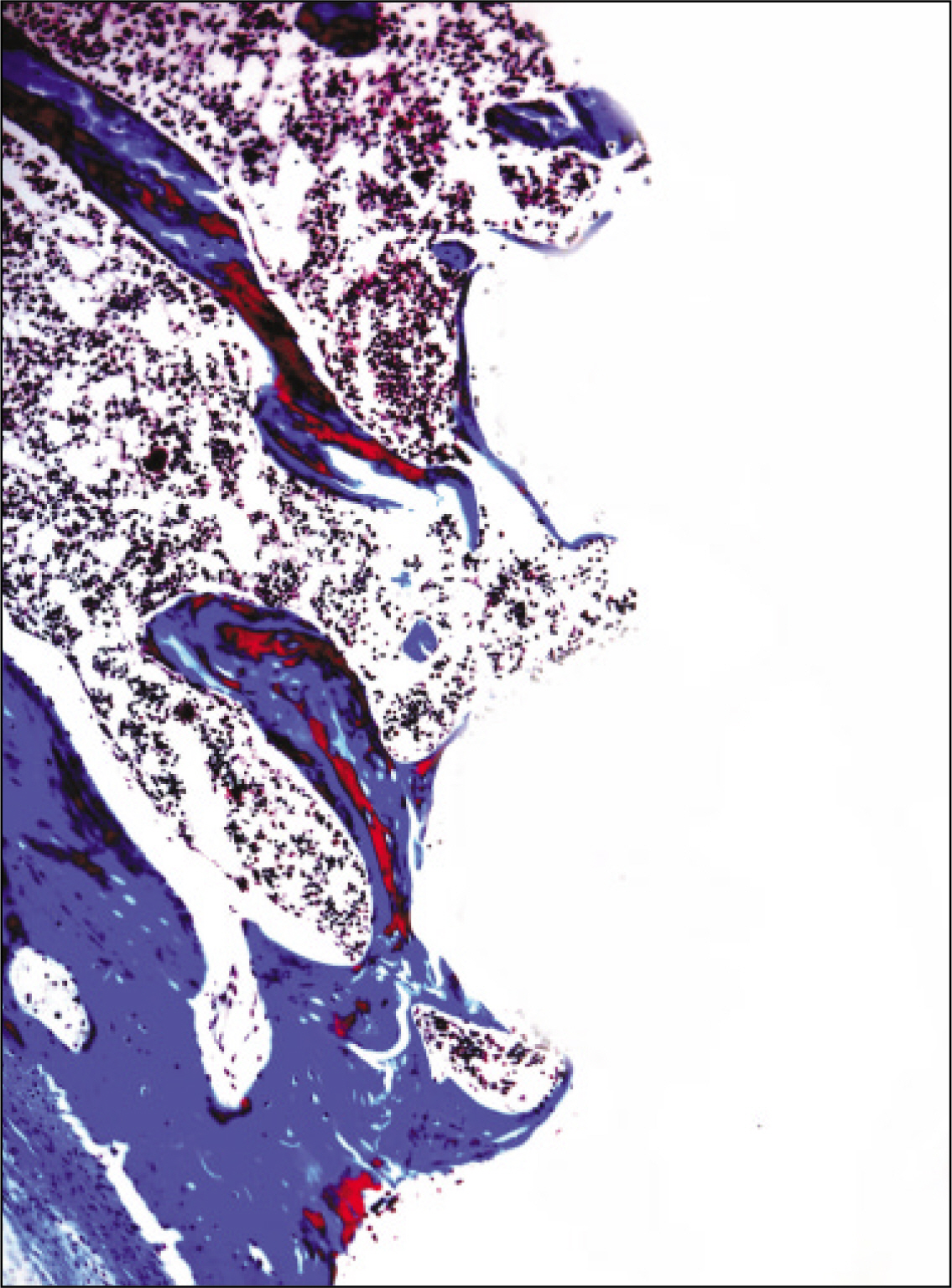
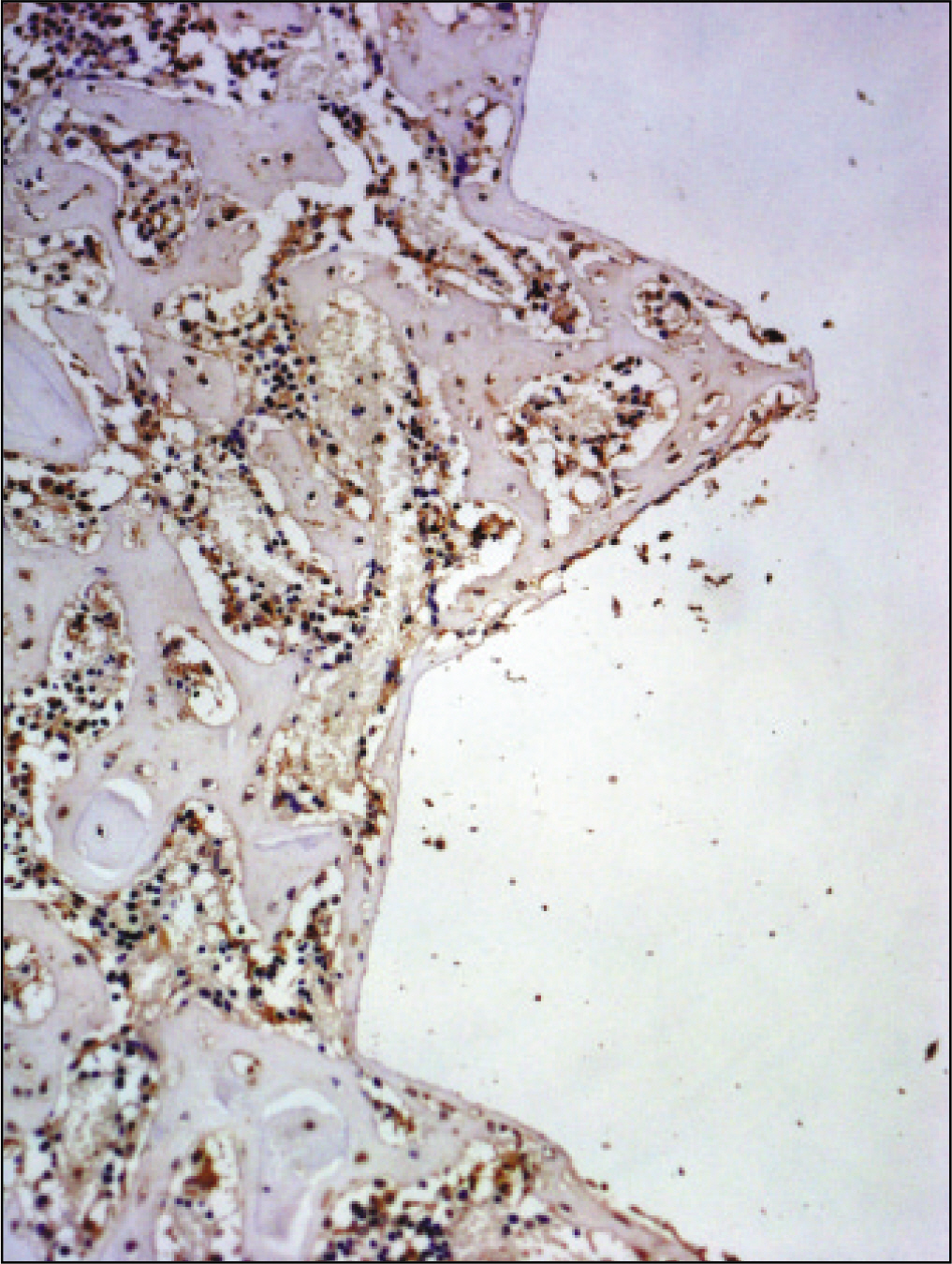
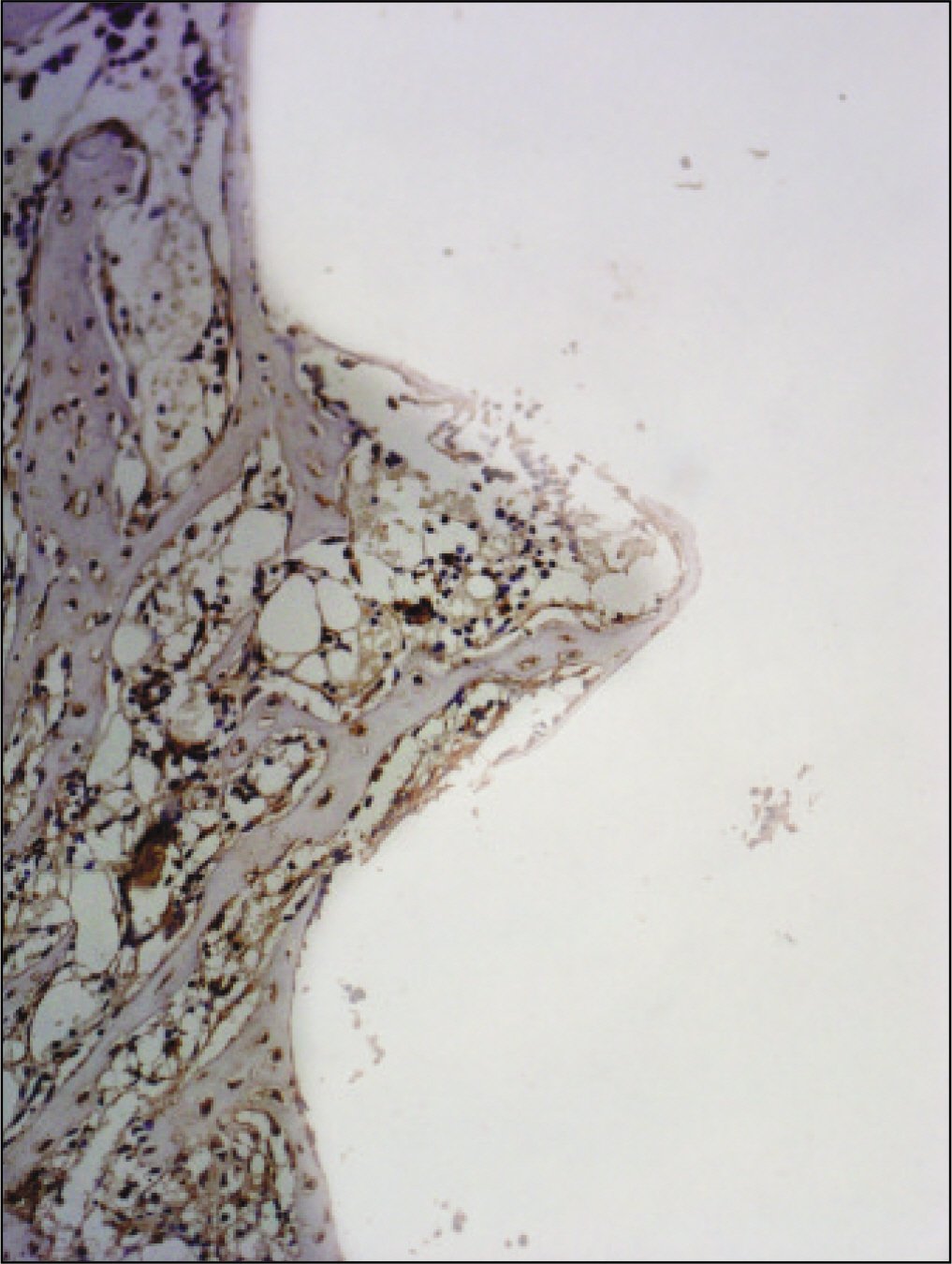
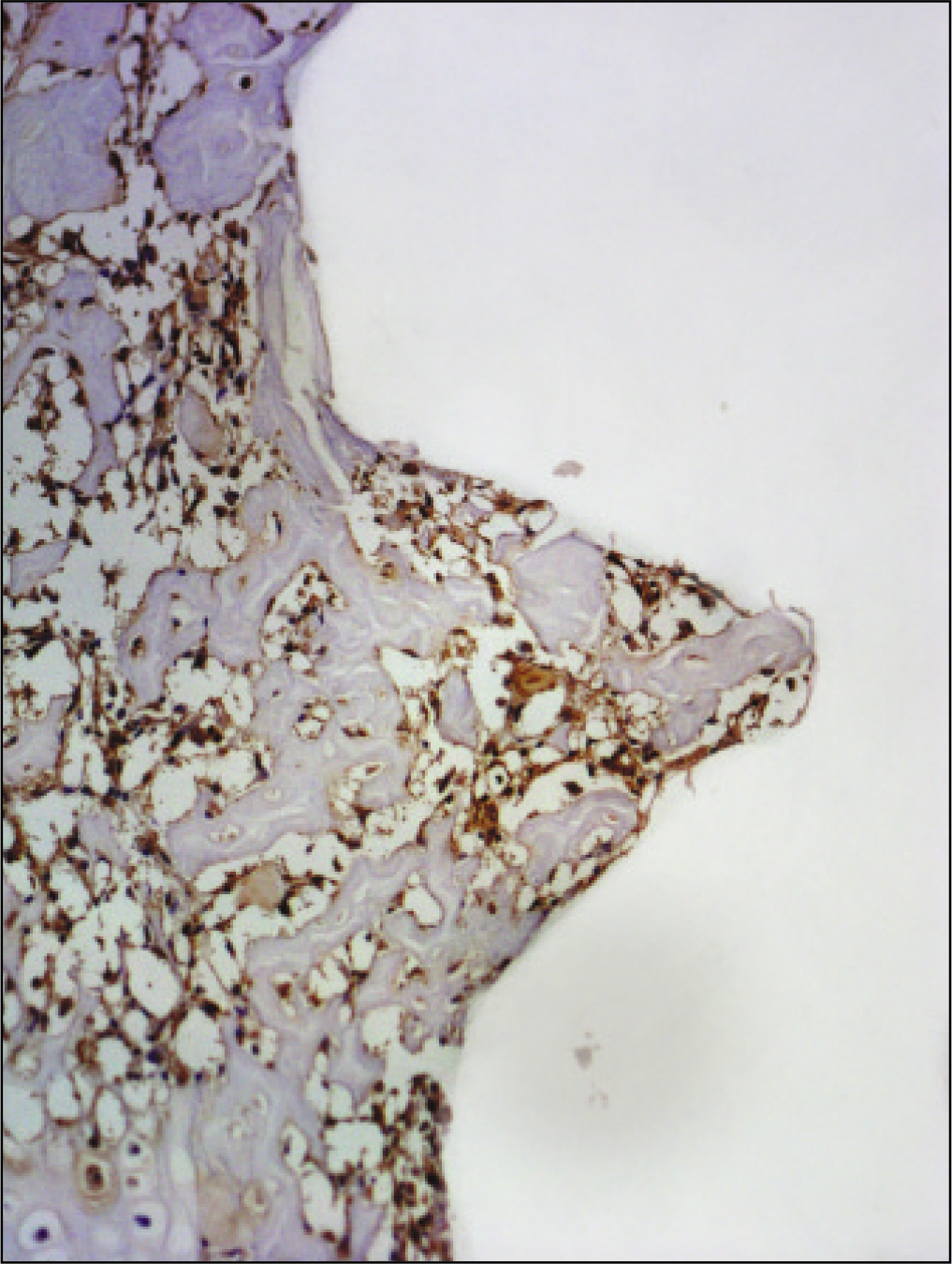
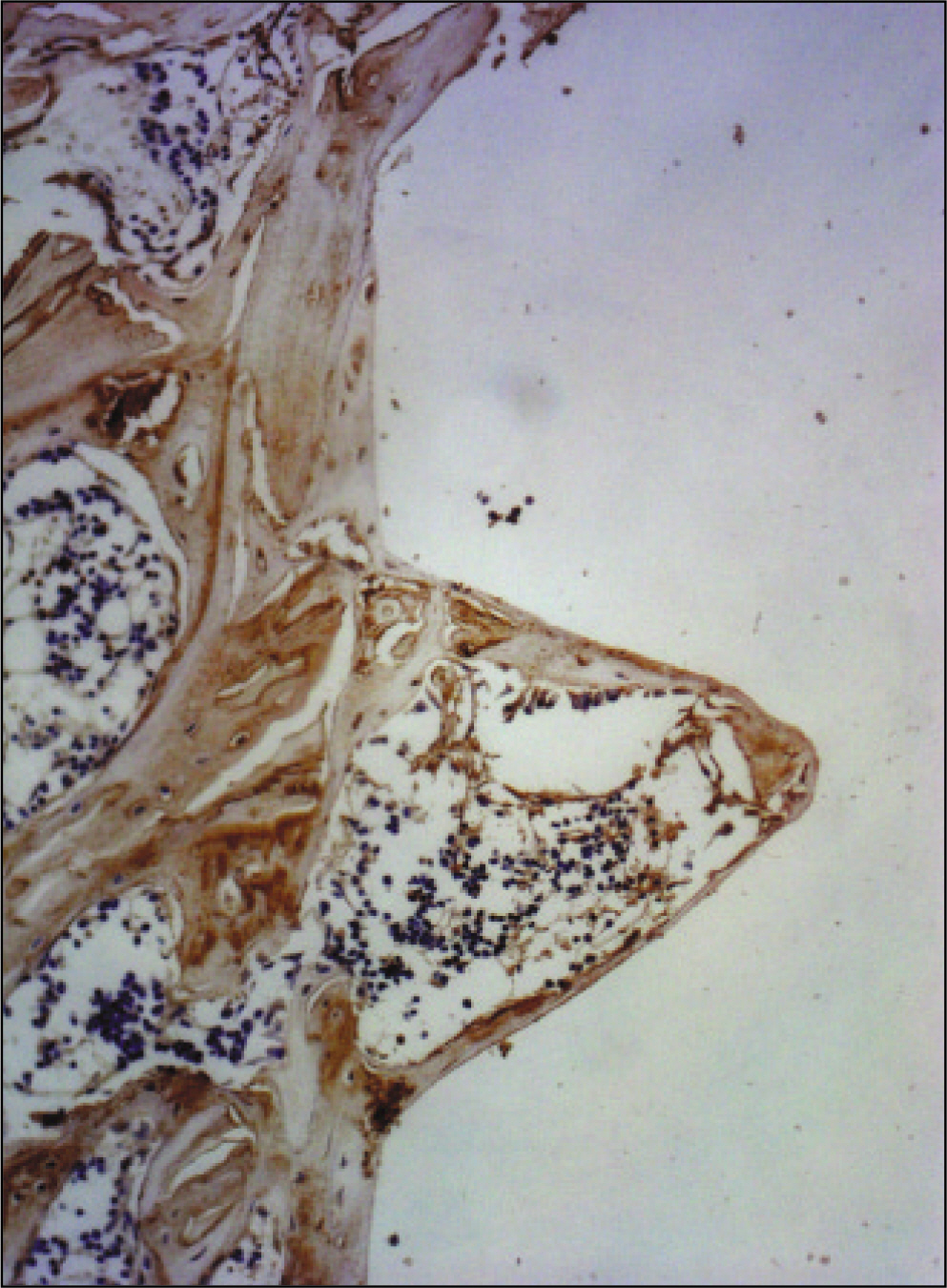
The histopathological findings revealed earlier new formed bone in the experimental group than the control group. In particular, at 1 week after implantation, the experimental group showed more newly formed bone and collagen around the implant than the control group. In immunohistochemistric analysis, osteoprotegerin (OPG) expression in the experimental group increased early compared to that of the control group until 2 weeks after implantation. However, after 2 weeks, OPG expression in the experimental group was similar to OPG expression in the control group. The receptor activator of nuclear factor kappaB ligand (RANKL) expression in the experimental group increased early compared to that of the control group, and then decreased at 2 weeks. After 2 weeks, the level of RANKL expression was similar in both groups.
CONCLUSION
These results suggest that adipose-derived stem cells in implantation can promote bone healing around titanium, particularly in diabetes mellitus induced animals.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Zimmet P, Shaw J, Alberti KG. Preventing type 2 diabetes and the dysmetabolic syndrome in the real world: a realistic view. Diabet Med. 2003; 20:693–702.
Article2. Weiss RE, Gora A, Nimni ME. Abnormalities in the biosynthesis of cartilage and bone proteoglycans in experimental diabetes. Diabetes. 1981; 30:670–7.
Article3. Goodman WT, Hori MT. Diminished bone formation in experimental diabetes. Relationship to osteoid maturation and mineralization. Diabetes. 1984; 33:825–31.
Article4. Schwartz Z, Somers A, Mellonig JT, Carnes DL Jr, Dean DD, Cochran DL, et al. Abillity of commercial demineralized freeze-dried bone allograft to induce new bone formation is dependent on donor age but gender. J Periodontol. 1998; 69:470–8.5. Traianedes K, Russell JL, Edwards JT, Stubbs HA, Shanahan IR, Knaack D. Donor age and gender effects on osteoinductivity of demineralized bone matrix. J Biomed Mater Res B Appl Biomater. 2004; 70:21–9.
Article6. Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974; 17:331–40.7. Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991; 9:641–50.
Article8. Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrowderived mesenchymal progenitor cells. Exp Cell Res. 1998; 238:265–72.9. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284:143–7.
Article10. Fukumoto T, Sperling JW, Sanyal A, Fitzsimmons JS, Reinholz GG, Conover CA, et al. Combined effects of insulinlike growth factor-1 and transforming growth factor-beta1 on periosteal mesenchymal cells during chondrogenesis in vitro. Osteoarthritis Cartilage. 2003; 11:55–64.11. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: Implications for cell based therapies. Tissue Eng. 2001; 7:211–28.12. de Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, et al. Comparison of multilineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003; 174:101–9.
Article13. Kwan Tat S, Padrines M, The ′ oleyre S, Heymann D, Fortun Y. Survey: IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004; 15:49–60.14. Theoleyre S, Wittrant Y, Tat SK, Fortun Y, Redini F, Heymann D. Survey: the molecular triad OPG/RANK/RANKL: involvement in the orchestration of pathophysiological bone remodeling. Cytokine Growth Factor Rev. 2004; 15:457–75.15. Boyle WJ, Simonet WS, Lecey DL. Osteoclast differentiation and activation. Nature. 2003; 423:337–42.
Article16. McCracken M, Lemons JE, Rahemtulla F, Prince CW, Feldman D. Bone response to titanium alloy implants placed in diabetic rats. Int J Oral Maxillofac Implants. 2000; 15:345–54.17. Nyomba BL, Verhaeghe J, Thomasset M, Lissens W, Bouillon R. Bone mineral homeostasis in spontaneously diabetic BB rats. Abnormal vitamin D metabolism and impaired active intestinal calcium absorption. Endocrinology. 1989; 124:565–72.18. Nevins ML, Karimbux NY, Weber HP, Giannobile WV, Fiorellini JP. Wound healing around endosseous implants in experimental diabetes. Int J Oral Maxillofac Implants. 1998; 13:620–9.
Article19. Morris HF, Ochi S, Winkler S. Implant survival in patients with type 2 diabetes: placement to 36 months. Ann Periodontol. 2000; 5:157–65.
Article20. Mellado-Valero A, Ferrer Garcl′a JC, Herrera Ballester A, Labaig Rueda C. Effects of diabetes on the osseointegration of dental implants. Med Oral Patol Oral Cir Bucal. 2007; 12:E38–43.21. Fiorellini JP, Chen PK, Nevins M, Nevins ML. A retrospective study of dental implants in diabetic patients. Int J Periodontics Restorative Dent. 2000; 20:366–73.22. Song L, Baksh D, Tuan RS. Mesenchymal stem cell-based cartilage tissue engineering: cells, scaffold and biology. Cytotherapy. 2004; 6:596–601.
Article23. Barry FP, Murphy JM. Mesenchymal stem cells: clinical application and biological characterization. Int J Biochem Cell Biol. 2004; 36:568–84.24. Kassem M, Kristiansen M, Abdallah BM. Mesenchymal stem cells: cell biology and potential use in therapy. Basic Clin Pharmacol Toxicol. 2004; 95:209–14.
Article25. Friedenstein AJ, Petrakova KV, Kurolesoval AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968; 6:230–47.26. Ohgushi H, Caplan AI. Stem cell technology and bioceramics: from cell to gene engineering. J Biomed Mater Res. 1999; 48:913–27.
Article27. Hofbauer LC, Heufelder AE. Role of receptor activator of nuclear factor-kappaB ligand and osteoprotegerin in bone cell biology. J Mol Med. 2001; 79:243–53.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Low Intensity Pulsed Ultrasound with Adipose-Derived Stem Cells on Bone Healing around a Titanium Implant in Tibia of Osteoporosis-Induced Rats
- Effect on bone healing by the application of low intensity pulsed ultrasound after injection of adipose tissue-derived stem cells at the implantation of titanium implant in the tibia of diabetes-induced rat
- Effect of Low-Intensity Pulsed Ultrasound on Bone Healing around Titanium Implant in Tibia of Diabetes Mellitus Induced Rats
- Effect of low intensity pulsed ultrasound (LIPUS) on bone healing around a titanium implant in the tibia of osteoporosis-induced rats
- Bone response around immediately placed titanium implant in the extraction socket of diabetic and insulin-treated rat maxilla