J Korean Assoc Oral Maxillofac Surg.
2011 Oct;37(5):386-395.
Effect of low intensity pulsed ultrasound (LIPUS) on bone healing around a titanium implant in the tibia of osteoporosis-induced rats
- Affiliations
-
- 1Department of Oral and Maxillofacial Surgery, School of Dentistry, Pusan National University, Yangsan, Korea. kuksjs@pusan.ac.kr
- 2Department of Oral Anatomy, School of Dentistry, Pusan National University, Yangsan, Korea.
- 3Department of Oral and Maxillofacial Surgery, Department of Dentistry, Dong-A University Medical Center, Pusan, Korea.
Abstract
- INTRODUCTION
Osteoporosis is a major health problem in the elderly that involves changes in the properties of bone as well as impaired bone healing around a titanium implant in both humans and animals. This study examined effect of low intensity pulsed ultrasound (LIPUS) on the bone healing process around a titanium implant in osteoporosis-induced rats.
MATERIALS AND METHODS
Sixteen rats were divided into two groups. A control group with osteoporosis induced by removing both ovaries and an experimental group of rats that were applied with LIPUS after osteoporosis had been induced. A screw type titanium implant (diameter, 2.0 mm: length, 3.5 mm, Cowell-Medi, KOREA) was placed into the tibias of 16 rats. The control and experimental group contained 8 rats each. The rats were sacrificed at 1, 2, 4, and 8 weeks after implantation to examine the histopathology and immunochemistry.
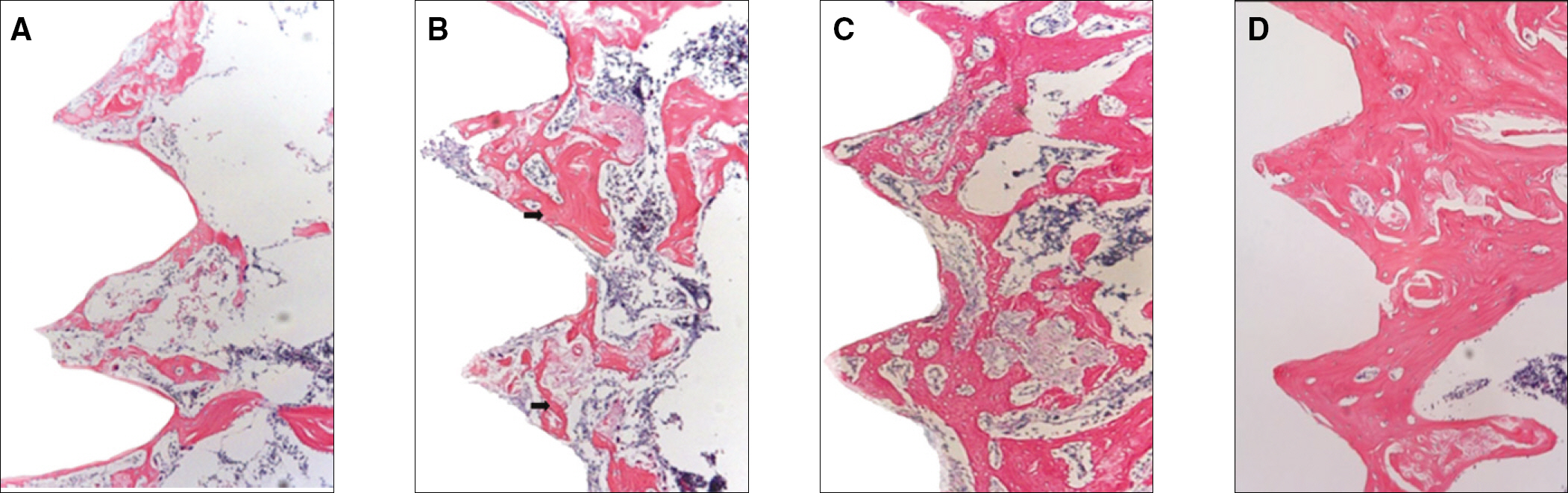
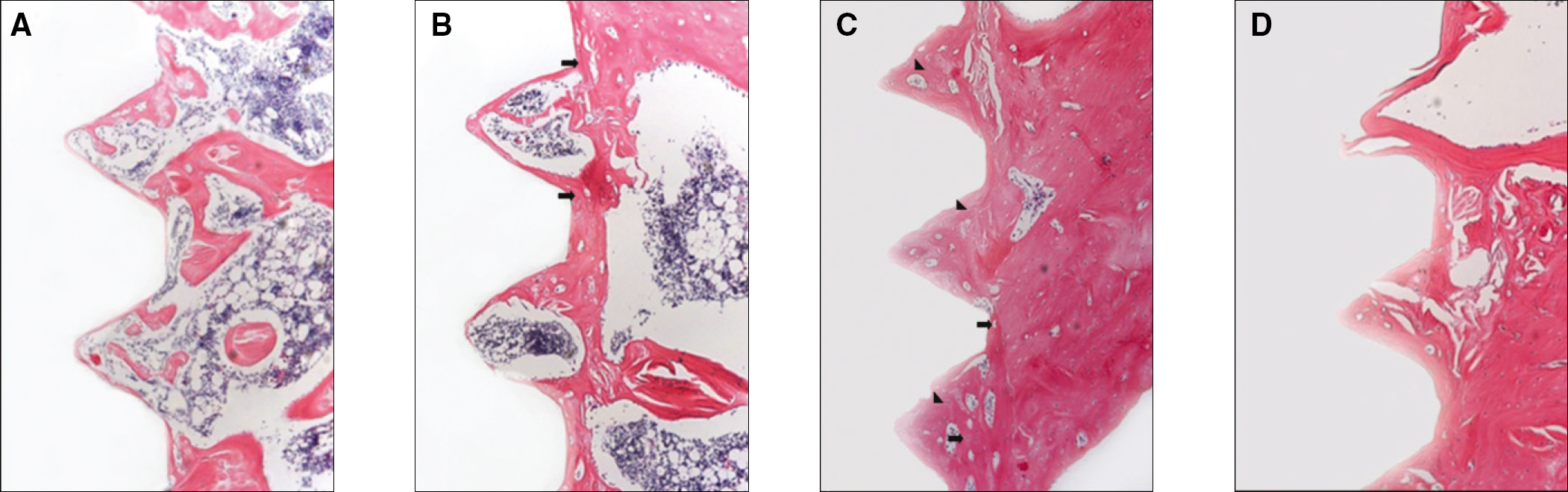
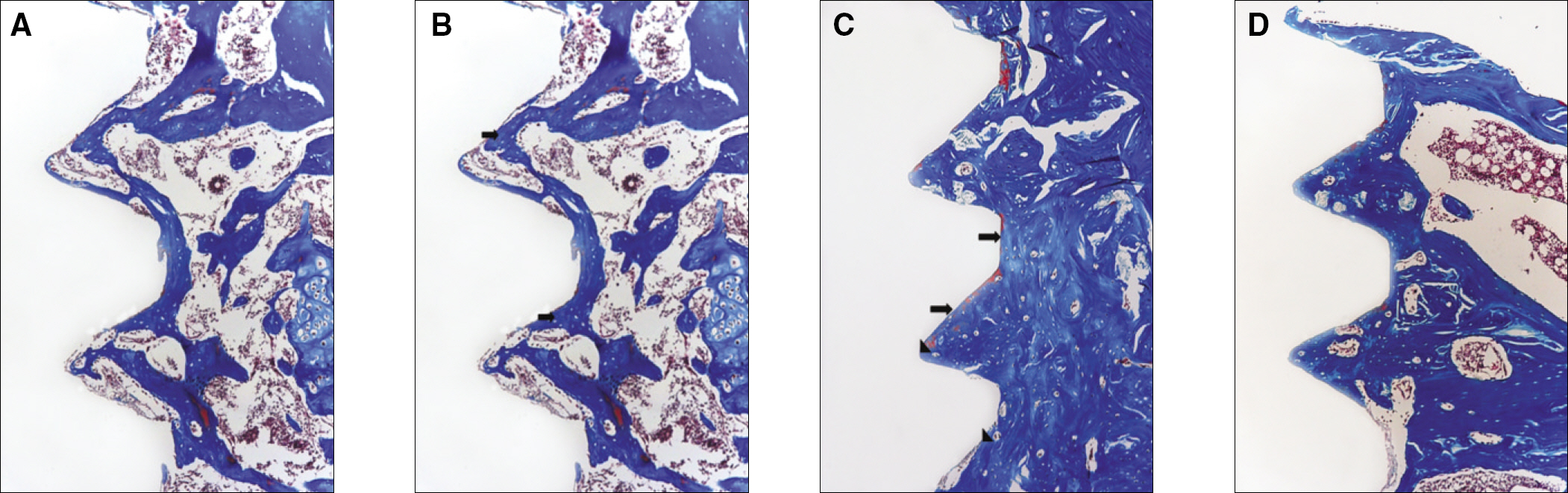
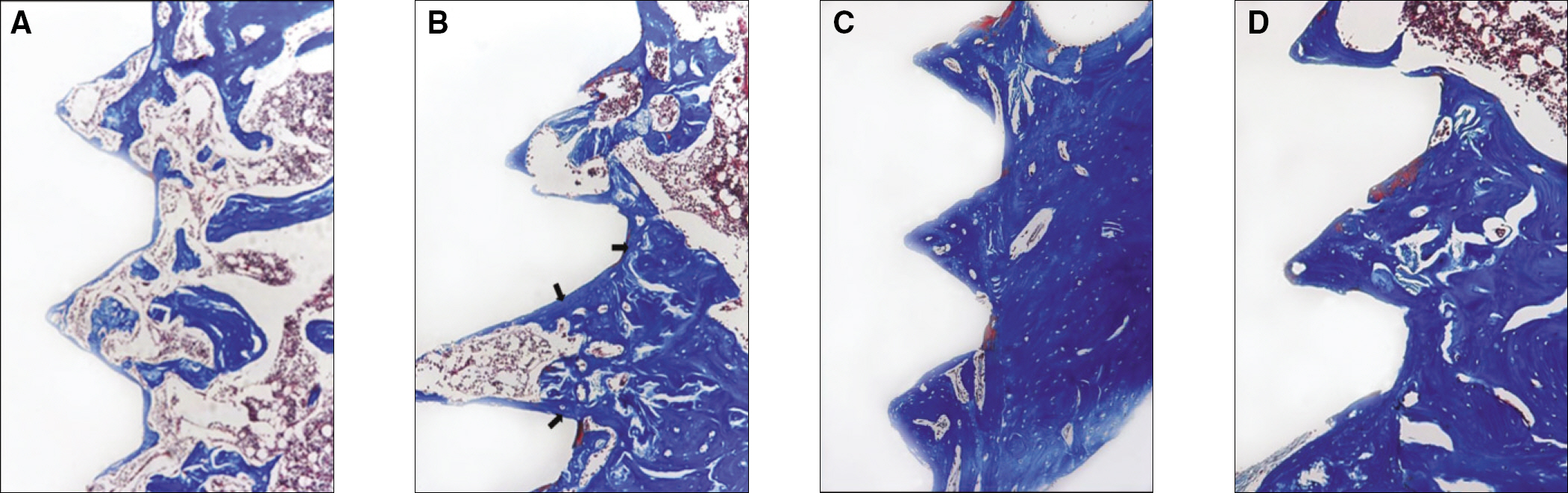
RESULTS
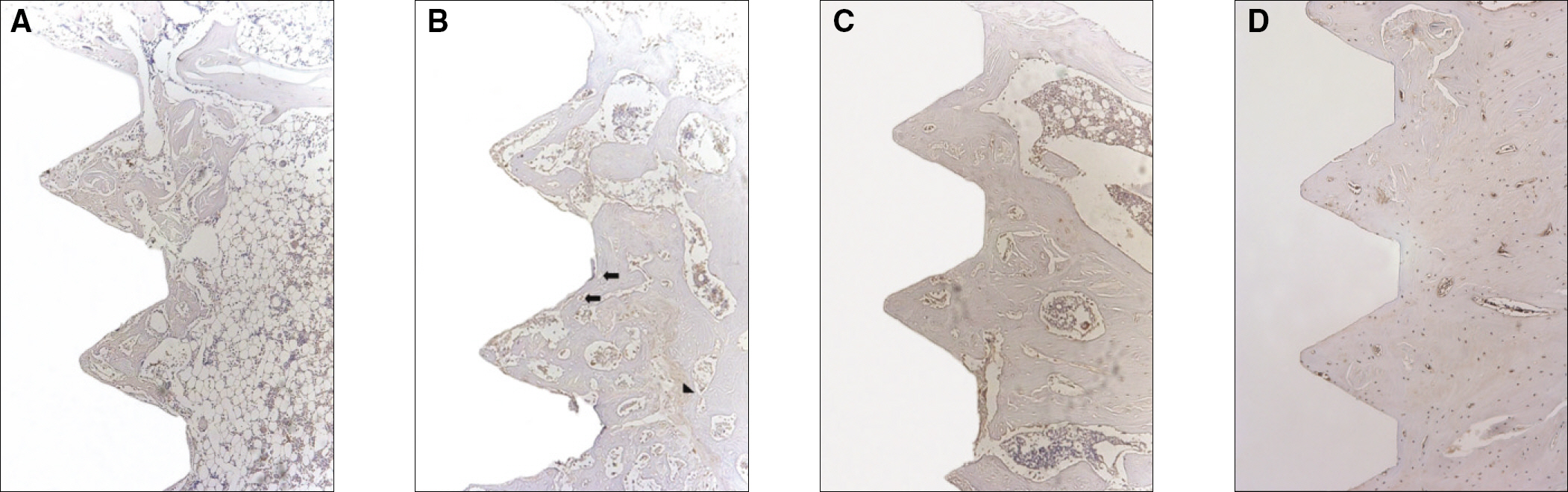
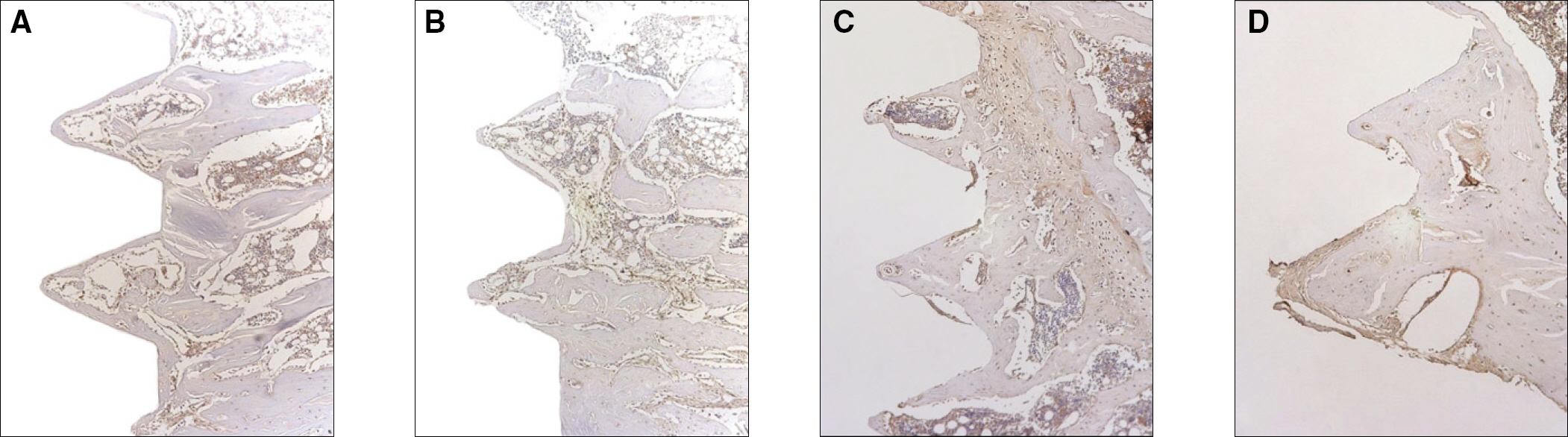
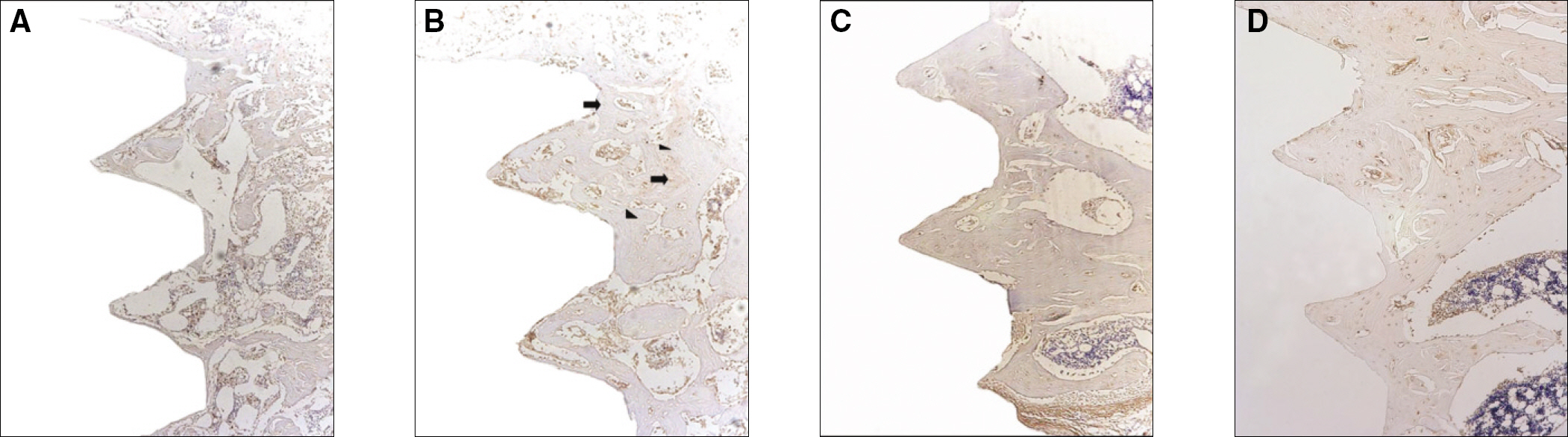
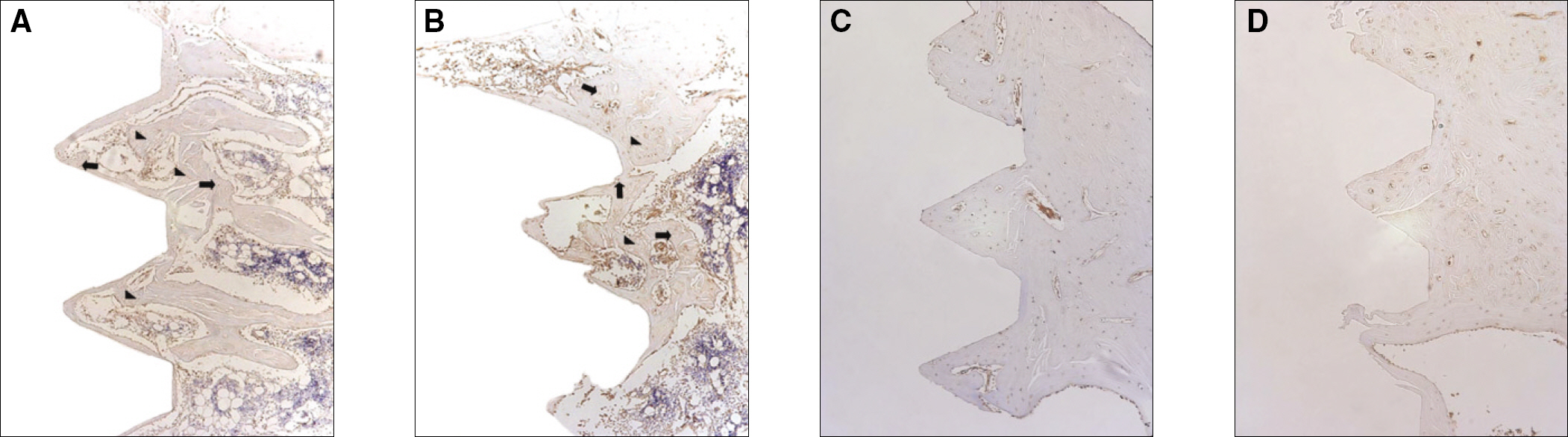
The histopathology examination revealed earlier new bone formation in the experimental group than the control group. In particular, at 1 week after implantation, more new bone matrix and collagen were observed around the implant of the experimental group compared to the control group. Immunochemistry analysis showed that the level of OPG expression of the experimental group was higher in the early stages than in the control group. After 8 weeks, the levels of OPG expression were similar in both groups. The expression level of receptor activator of nuclear factor kB ligand (RANKL) was stronger in the experimental group than the control group. After 4 weeks, the level of RANKL expression was similar in both groups.
CONCLUSION
These results suggest that the application of LIPUS to implantation can promote bone healing around titanium in osteoporosis animals.
MeSH Terms
Figure
Reference
-
References
1. Lane JM, Russell L, Khan SN. Osteoporosis. Clin Orthop Relat Res. 2000; 372:139–150.
Article2. Siebzehner MI. Consensus statement on prevention and treatment of osteoporosis. Isr Med Assoc J. 2000; 2:397–401.3. Bagi CM, Mecham M, Weiss J, Miller SC. Comparative morphometric changes in rat cortical bone following ovariectomy and/or immobilization. Bone. 1993; 14:877–883.
Article4. Croucher PI, Garrahan NJ, Compston JE. Structural mechanisms of trabecular bone loss in primary osteoporosis: specific disease mechanism or early aging? Bone Miner. 1994; 25:111–121.5. Jiang G, Matsumoto H, Fujii A. Mandible bone loss in osteoporosis rats. J Bone Miner Metab. 2003; 21:388–395.
Article6. Cao T, Shirota T, Ohno K, Michi KI. Mineral bone loss in partially edentul ous trabeculae of ovariectomized rabbit mandibles. J Periodontal Res. 2004; 39:37–41.7. Yang J, Farnell D, Devlin H, Horner K, Graham J. The effect of ovariectomy on mandibular cortical thickness in the rat. J Dent. 2005; 33:123–129.
Article8. Dimitriou R, Babis GC. Biomaterial osseointegration Enhancement with biophysical stimulation. J Musculoskelet Neuronal Interact. 2007; 7:253–265.9. Grassi S, Piattelli A, Ferrari DS, Figueiredo LC, Feres M, Iezzi G, et al. Histologic evaluation of human bone integration on machined and sandblasted acid-etched titanium surfaces in type IV bone. J Oral Implantol. 2007; 33:8–12.
Article10. Shibli JA, Grassi S, de Figueiredo LC, Feres M, Marcantonio E Jr, Iezzi G, et al. Influence of implant surface topography on early osseointegration: a histological study in human jaws. J biomed Mater Res B Appl Biomater. 2007; 80:377–385.
Article11. Li L, Zhu Z, Huang C, Chen W. Ultrasound: a potential technique to improve ossseointegration of dental implants. Med Hypotheses. 2008; 71:568–571.12. Takayama B, Suzuki N, Ikeda K, Shimada T, Suzuki A, Maeno M, et al. Low-intensity pulsed ultrasound stimulates osteogenic differentiation in ROS 17/2.8 cells. Life Sci. 2007; 80:965–971.
Article13. Buckly MJ, Banes AJ, Levin LG, Sumpio BE, Sato M, Jordan R, et al. Osteoblasts increase their rate of division and in response to cyclic, mechanical tension in vitro. Bone Miner. 1988; 4:225–236.14. Harle J, Salih V, Mavia F, Knowles JC, Olsen I. Effects of ultrasound on the growth and function of bone and periodontal ligament cells in vitro. Ultrasound Med Biol. 2001; 27:579–586.
Article15. Saito M, Soshi S, Tanaka T, Fujii K. Intensity related differences in collagen post-translational modification in MC3T3-E1 osteoblasts after exposure to low- and high-intensity pulsed ultrasound. Bone. 2004; 35:644–655.16. Lyon R, Liu XC, Meier J. The effects of therapeutic vs. high intensity ultrasound in the rabbit growth plate. J orthop Res. 2003; 21:865–871.17. Heckman JD, Ryaby JP, McCabe j, Frey JJ, Kilcoyne RF. Acceleration of tibial fracture-healing by non-invasive, low intensity pulsed ultrasound. J Bone Joint Surg Am. 1994; 76:26–34.18. Kristiansen TK, Ryaby JP, McCabe J, Frey JJ, Roe LR. Accelerated healing of distalradial fractures with the use if specific, low-intensity ultrasound. A multicenter, prospective, randomized, double-blind, placebo-controlled study. J Bone Joint Surg Am. 1997; 79:961–973.19. Leung KS, Lee WS, Tsui HF, Liu PP, Cheung WH. Complex tibial frature outcomes following treatment with low intensity pulsed ultrasound. Ultrasound Med Biol. 2004; 30:389–395.20. Yang RS, Lin WL, Chen YZ, Tang CH, Huang TH, Lu BY, et al. Regulation by ultrasound treatment on the integrin expression and differentiation of osteoblasts. Bone. 2005; 36:276–283.
Article21. Yang KH, Parvizi J, Wang SJ, Lewallen DG, Kinnick RR, Greenleaf JF, et al. Exposure to low-intensity ultrasound increases aggrecan gene expression in a rat femur fracture model. J Orthop Res. 1996; 14:802–809.
Article22. Parvizi J, Parpura V, Greenleaf JF, Bolander ME. Calcium signaling is required for ultrasound-stimulated aggrecan synthesis by rat chondrocytes. J Orthop Res. 2002; 20:51–57.
Article23. Parvizi J, Wu CC, Lewallen DG, Greenleaf JF, Bolander . Low-intensity ultrasound stimulates proteoglycan synthesis in rat chondrocytes by increasing aggrecan gene expression. J Orthop Res. 1999; 17:488–94.
Article24. Naruse K, Mikuni-Takagaki Y, Azuma Y, Ito M, Oota T, Kameyama K, et al. Anabolic response of mouse bone marrowderived stromal cell clone ST2 cells to low-intensity pulsed ultrasound. Biochem Biophys Res Common. 2000; 268:216–220.25. Naruse K, Miyauchi A, Itoman M, Mikuni-Takagaki Y. Distinct anabolic response of osteoblast to low-intensity pulsed ultrasound. J Bone Miner Res. 2003; 18:360–369.
Article26. Wang SJ, Lewallen DG, Bolander ME, Chao EY, Ilstrup DM, Greenleaf JF. Low intensity ultrasound treatment increases strength in a rat femoral fracture model. J Orthop Res. 1994; 12:40–47.
Article27. Diego Araujo DB, Eiji T, Toshihiro I, Oka H, Ohta A, Okada H, et al. Cementoblast response to low- and high-intensity ultrasound. Archives of Oral Biology. 2008; 53:318–323.
Article28. Ganne JM, Speculand B, Mayne KH, Goss AN. Inferential therapy to promote union of mandibular fractures. Aust N Z J Surg 199;49:. 81–83.29. Kubota K, Yoshimura N, Yakota M, Fitzsimmons RJ, Wikesjo¨ ME. Overview of effects of electrical stimulation on osteogenesis and alveolar bone. J Periodontol. 1995; 66:2–6.
Article30. Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO study group. Osteoporosis Int. 1994; 4:368–381.31. Kanis JA, Melton LJ III, Christiansen C, et al. The diagnosis of osteoporosis. J Bone Miner Res. 1994; 9:1137–1141.
Article32. Holahan CM, Koka S, Kennel KA, Weaver AL, Assad DA, Regennitter FJ, et al. Effect of osteoporotic status on the survival of titanium dental implants. Int J Oral Maxillofac Implants. 2009; 23:905–910.33. Friberg B, Ekestubbe A, Mellstrom D, Sennerby L. Bra¨nemark Implants and Osteoporosis. A Clinical Exploratory Study. Clin Implant Dent Relat Res. 2001; 3:50–56.34. Robert WE, Simons KE. Garetto LP, DeCastro RA. Bone physiology and metabolism in dental implantology: Risk Factors for osteoporosis and other metabolic bone disease. Implant Dent. 1992; 1:11–21.35. Erdogan O, Shafer DM, Taxel P, Freilich MA. A review of the association between osteoporosis and alveolar ridge augmentation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007; 104:738. .e1–13.36. Machen MS, Tis JE, Inoue N, Meffert RH, Chao EY, McHale KA. The effect of low intensity pulsed ultrasound in regenerate bone in a less-than-rigid biomechanical environment. Bio Med Mater and Eng. 2002; 12:239–247.37. Azuma Y, Ito M, Harada Y, Takagi H, Ohta T, Jingushi S. Low-intensity pulsed ultrasound accelerates rat femoral frature healing by acting on the various cellular reaction in the fracture callus. J Bone Miner Res. 2001; 16:671–680.38. Doan N, Reher P, Meghji S, Harris M. In vitro effects of therapeutic ultrasound on cell proliferation, protein synthesis, and cytokine production by human fibroblasts, osteoblasts, and monocytes. J Oral Maxillofac Surg. 1999; 57:409–419.
Article39. Kwan Tat S, Padrines M, Theoleyres S Heymann D, Fortun Y. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004; 15:49–60.40. Hofbaur LC, Heufelder AE. Role of receptor activator of nuclear factor-λ B ligand and osteoprotegerin in bone cell biology. J Mol Med. 2001; 79:243–253.41. Hakeda Y, Kobayashi Y, Yamaguchi K, Yasuda H, Tsuda E, Higashio K, et al. Osteoclastogenesis inhibitory factor (OCIF) directly inhibits bone-resorbing activity of isolated mature osteoclasts. Biochem Biophys Res Commun. 1998; 251:796–801.
Article42. Theoleyre S, Wittrant Y, Tat SK, Fortun Y, Redini F, Heymann D. The molecular triad OPG/RANK/RANKL: involvement in the orchestration of pathophysiologic bone remodeling. Cytokine & Growth Factor Reviews. 2004; 15:457–475.43. Kim M-K, Chung I-K, Shin S-H, Kim C-H, Kim B-J, Kim J-H, Hwang Y-S, Jung E-G, Kim J-H, Kim U-K. Effect of Adipose-Derived Stem Cells on Bone Healing on Titanium Implant in Tibia of Diabetes Mellitus Induced Rats, J Kor Assoc Oral Maxillofac Surg. 2010; 36:392–401.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Low Intensity Pulsed Ultrasound with Adipose-Derived Stem Cells on Bone Healing around a Titanium Implant in Tibia of Osteoporosis-Induced Rats
- Effect of Low-Intensity Pulsed Ultrasound on Bone Healing around Titanium Implant in Tibia of Diabetes Mellitus Induced Rats
- Effects of combined therapy of alendronate and low-intensity pulsed ultrasound on metaphyseal bone repair after osteotomy in the proximal tibia of glucocorticoid-induced osteopenia rats
- Effect on bone healing by the application of low intensity pulsed ultrasound after injection of adipose tissue-derived stem cells at the implantation of titanium implant in the tibia of diabetes-induced rat
- Enhanced Bone Regeneration by Bone Morphogenetic Protein-2 after Pretreatment with Low-Intensity Pulsed Ultrasound in Distraction Osteogenesis