Tuberc Respir Dis.
2015 Oct;78(4):315-320. 10.4046/trd.2015.78.4.315.
The Prognostic Value of the Tumor Shrinkage Rate for Progression-Free Survival in Patients with Non-Small Cell Lung Cancer Receiving Gefitinib
- Affiliations
-
- 1Division of Pulmonary, Department of Internal Medicine, Chungnam National University Hospital, Daejeon, Korea. vov-x@daum.net
- 2Department of Radiology, Chungnam National University Hospital, Daejeon, Korea.
- 3Department of Preventive Medicine, Chungnam National University Hospital, Daejeon, Korea.
- KMID: 2320699
- DOI: http://doi.org/10.4046/trd.2015.78.4.315
Abstract
- BACKGROUND
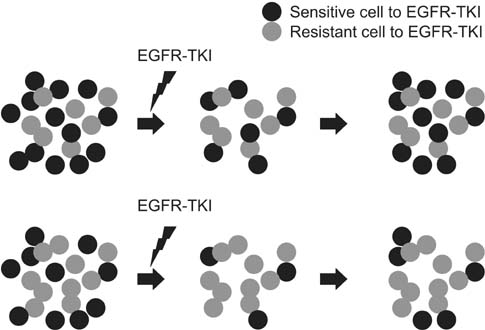
The efficacy of epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) therapy can be measured based on the rate of treatment response, based on the Response Evaluation Criteria in Solid Tumors (RECIST) criteria or progression-free survival (PFS). However, there are some patients harboring sensitive EGFR mutations who responded poorly to EGFR-TKI therapy. In addition, there is variability in the PFS after EGFR-TKI treatment.
METHODS
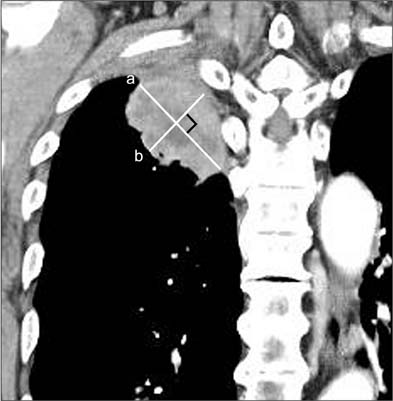
We performed a retrospective analysis of the medical records of 85 patients with non-small cell lung cancer, who had achieved a stable disease or better response at the first evaluation of treatment response, after receiving a 2-month course of gefitinib. We calculated the tumor shrinkage rate (TSR) by measuring the longest and perpendicular diameter of the main mass on computed tomography before, and 2 months after, gefitinib therapy.
RESULTS
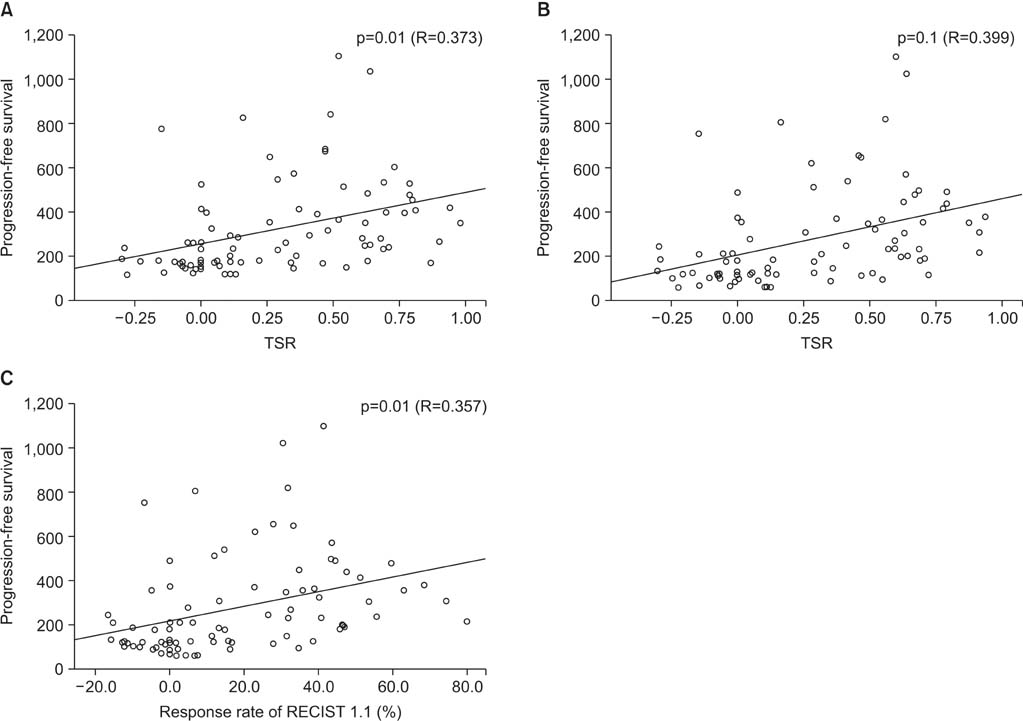
There was a significant positive correlation between the TSR and PFS (R=0.373, p=0.010). In addition, a simple linear regression analysis showed that the TSR might be an indicator for the PFS (B+/-standard error, 244.54+/-66.79; p=0.001). On univariate analysis, the sex, histologic type, smoking history and the number of prior chemotherapy regimens, were significant prognostic factors. On multivariate regression analysis, both the TSR (beta=0.257, p=0.029) and adenocarcinoma (beta=0.323, p=0.005) were independent prognostic factors for PFS.
CONCLUSION
Our results showed that the TSR might be an early prognostic indicator for PFS in patients receiving EGFR-TKI therapy.
Keyword
MeSH Terms
Figure
Reference
-
1. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009; 361:947–957.2. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-smallcell lung cancer with mutated EGFR. N Engl J Med. 2010; 362:2380–2388.3. Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010; 11:121–128.4. Han JY, Park K, Kim SW, Lee DH, Kim HY, Kim HT, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012; 30:1122–1128.5. Chen ZY, Zhong WZ, Zhang XC, Su J, Yang XN, Chen ZH, et al. EGFR mutation heterogeneity and the mixed response to EGFR tyrosine kinase inhibitors of lung adenocarcinomas. Oncologist. 2012; 17:978–985.6. Taniguchi K, Okami J, Kodama K, Higashiyama M, Kato K. Intratumor heterogeneity of epidermal growth factor receptor mutations in lung cancer and its correlation to the response to gefitinib. Cancer Sci. 2008; 99:929–935.7. Michor F, Polyak K. The origins and implications of intratumor heterogeneity. Cancer Prev Res (Phila). 2010; 3:1361–1364.8. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011; 12:735–742.9. Gridelli C, Rossi A. EURTAC first-line phase III randomized study in advanced non-small cell lung cancer: erlotinib works also in European population. J Thorac Dis. 2012; 4:219–220.10. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated nonsmall-cell lung cancer. N Engl J Med. 2005; 353:123–132.11. Oxnard GR, Arcila ME, Chmielecki J, Ladanyi M, Miller VA, Pao W. New strategies in overcoming acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in lung cancer. Clin Cancer Res. 2011; 17:5530–5537.12. Chmielecki J, Foo J, Oxnard GR, Hutchinson K, Ohashi K, Somwar R, et al. Optimization of dosing for EGFR-mutant non-small cell lung cancer with evolutionary cancer modeling. Sci Transl Med. 2011; 3:90ra59.13. Nguyen KS, Kobayashi S, Costa DB. Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancers dependent on the epidermal growth factor receptor pathway. Clin Lung Cancer. 2009; 10:281–289.14. Oh IJ, Ban HJ, Kim KS, Kim YC. Retreatment of gefitinib in patients with non-small-cell lung cancer who previously controlled to gefitinib: a single-arm, open-label, phase II study. Lung Cancer. 2012; 77:121–127.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A case of leptomeningeal metastasis from adenocarcinoma of the lung improved by treatment with Gefitinib
- The Effectiveness of Gefitinib on Spinal Metastases of Lung Cancer: Report of Two Cases
- Post-Progression Survival in Patients with Non-Small Cell Lung Cancer with Clinically Acquired Resistance to Gefitinib
- The Relationship between (the) Loss of Blood Group Antigen A in Cancer Tissue and Survival Time in the Antigen A Positive Non-Small Cell Lung Cancer
- Comparison of Gefitinib and Erlotinib for Patients with Advanced Non-Small-Cell Lung Cancer