Evaluation of reparative dentin formation of ProRoot MTA, Biodentine and BioAggregate using micro-CT and immunohistochemistry
- Affiliations
-
- 1Department of Conservative Dentistry, School of Dentistry, Dental Science Research Institute, Chonnam National University, Gwangju, Korea. ychwang@chonnam.ac.kr
- 2Department of Conservative Dentistry, School of Dentistry, Chonbuk National University, Jeonju, Korea.
- 3Research Center for Biomineralization Disorders, Chonnam National University, Gwangju, Korea.
- KMID: 2316961
- DOI: http://doi.org/10.5395/rde.2016.41.1.29
Abstract
OBJECTIVES
The purpose of this study was to assess the ability of two new calcium silicate-based pulp-capping materials (Biodentine and BioAggregate) to induce healing in a rat pulp injury model and to compare them with mineral trioxide aggregate (MTA).
MATERIALS AND METHODS
Eighteen rats were anesthetized, cavities were prepared and the pulp was capped with either of ProRoot MTA, Biodentine, or BioAggregate. The specimens were scanned using a high-resolution micro-computed tomography (micro-CT) system and were prepared and evaluated histologically and immunohistochemically using dentin sialoprotein (DSP).
RESULTS
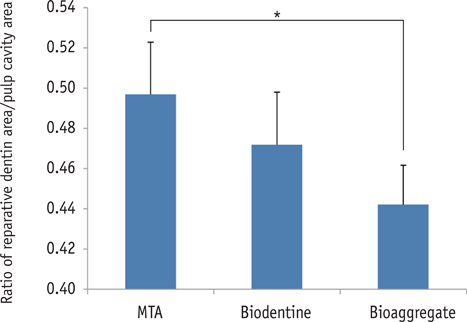
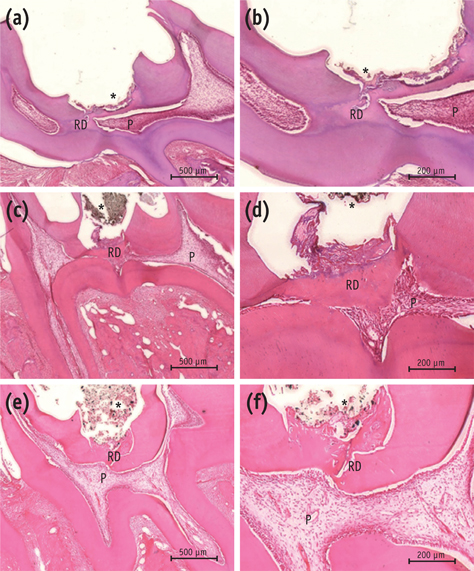
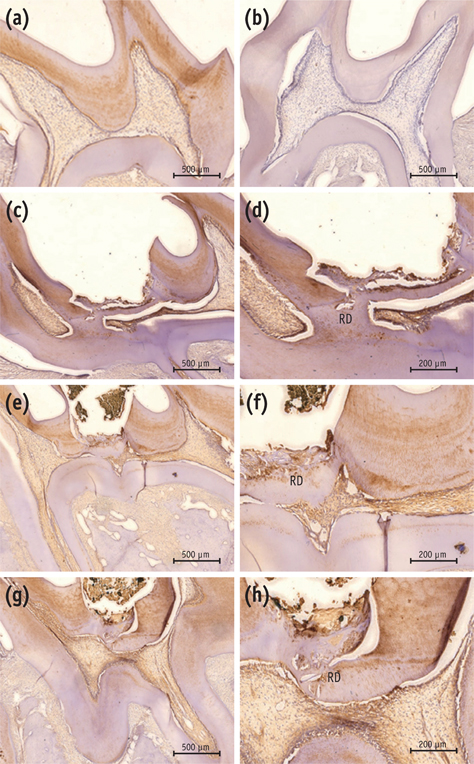
On micro-CT analysis, the ProRoot MTA and Biodentine groups showed significantly thicker hard tissue formation (p < 0.05). On H&E staining, ProRoot MTA showed complete dentin bridge formation with normal pulpal histology. In the Biodentine and BioAggregate groups, a thick, homogeneous hard tissue barrier was observed. The ProRoot MTA specimens showed strong immunopositive reaction for DSP.
CONCLUSIONS
Our results suggest that calcium silicate-based pulp-capping materials induce favorable effects on reparative processes during vital pulp therapy and that both Biodentine and BioAggregate could be considered as alternatives to ProRoot MTA.
Keyword
Figure
Cited by 3 articles
-
Micro-computed tomographic evaluation of the flow and filling ability of endodontic materials using different test models
Fernanda Ferrari Esteves Torres, Juliane Maria Guerreiro-Tanomaru, Gisselle Moraima Chavez-Andrade, Jader Camilo Pinto, Fábio Luiz Camargo Villela Berbert, Mario Tanomaru-Filho
Restor Dent Endod. 2020;45(2):e11. doi: 10.5395/rde.2020.45.e11.Micro-computed tomographic evaluation of a new system for root canal filling using calcium silicate-based root canal sealers
Mario Tanomaru-Filho, Fernanda Ferrari Esteves Torres, Jader Camilo Pinto, Airton Oliveira Santos-Junior, Karina Ines Medina Carita Tavares, Juliane Maria Guerreiro-Tanomaru
Restor Dent Endod. 2020;45(3):e34. doi: 10.5395/rde.2020.45.e34.Hard tissue formation after direct pulp capping with osteostatin and MTA
in vivo
Ji-Hye Yoon, Sung-Hyeon Choi, Jeong-Tae Koh, Bin-Na Lee, Hoon-Sang Chang, In-Nam Hwang, Won-Mann Oh, Yun-Chan Hwang
Restor Dent Endod. 2021;46(2):e17. doi: 10.5395/rde.2021.46.e17.
Reference
-
1. Dominguez MS, Witherspoon DE, Gutmann JL, Opperman LA. Histological and scanning electron microscopy assessment of various vital pulp-therapy materials. J Endod. 2003; 29:324–333.
Article2. Torabinejad M, Chivian N. Clinical applications of mineral trioxide aggregate. J Endod. 1999; 25:197–205.
Article3. de Souza V, Holland R. Treatment of the inflamed dental pulp. Aust Dent J. 1974; 19:191–196.
Article4. Eskandarizadeh A, Shahpasandzadeh MH, Shahpasandzadeh M, Torabi M, Parirokh M. A comparative study on dental pulp response to calcium hydroxide, white and grey mineral trioxide aggregate as pulp capping agents. J Conserv Dent. 2011; 14:351–355.
Article5. Faraco IM Jr, Holland R. Response of the pulp of dogs to capping with mineral trioxide aggregate or a calcium hydroxide cement. Dent Traumatol. 2001; 17:163–166.
Article6. Accorinte ML, Loguercio AD, Reis A, Carneiro E, Grande RH, Murata SS, Holland R. Response of human dental pulp capped with MTA and calcium hydroxide powder. Oper Dent. 2008; 33:488–495.
Article7. Zarrabi MH, Javidi M, Jafarian AH, Joushan B. Histologic assessment of human pulp response to capping with mineral trioxide aggregate and a novel endodontic cement. J Endod. 2010; 36:1778–1781.
Article8. Torabinejad M, Hong CU, Pitt Ford TR, Kaiyawasam SP. Tissue reaction to implanted super-EBA and mineral trioxide aggregate in the mandible of guinea pigs: a preliminary report. J Endod. 1995; 21:569–571.
Article9. Moretton TR, Brown CE Jr, Legan JJ, Kafrawy AH. Tissue reactions after subcutaneous and intraosseous implantation of mineral trioxide aggregate and ethoxybenzoic acid cement. J Biomed Mater Res. 2000; 52:528–533.
Article10. Kettering JD, Torabinejad M. Investigation of mutagenicity of mineral trioxide aggregate and other commonly used root-end filling materials. J Endod. 1995; 21:537–542.
Article11. Torabinejad M, Watson TF, Pitt Ford TR. Sealing ability of a mineral trioxide aggregate when used as a root end filling material. J Endod. 1993; 19:591–595.
Article12. Lee SJ, Monsef M, Torabinejad M. Sealing ability of a mineral trioxide aggregate for repair of lateral root perforations. J Endod. 1993; 19:541–544.
Article13. Schwartz RS, Mauger M, Clement DJ, Walker WA 3rd. Mineral trioxide aggregate: a new material for endodontics. J Am Dent Assoc. 1999; 130:967–975.
Article14. Holland R, de Souza V, Nery MJ, Faraco Júnior IM, Bernabé PF, Otoboni Filho JA, Dezan Júnior E. Reaction of rat connective tissue to implanted dentin tube filled with mineral trioxide aggregate, Portland cement or calcium hydroxide. Braz Dent J. 2001; 12:3–8.15. Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review-part III: clinical applications, drawbacks, and mechanism of action. J Endod. 2010; 36:400–413.
Article16. Jang YE, Lee BN, Koh JT, Park YJ, Joo NE, Chang HS, Hwang IN, Oh WM, Hwang YC. Cytotoxicity and physical properties of tricalcium silicate-based endodontic materials. Restor Dent Endod. 2014; 39:89–94.
Article17. Zanini M, Sautier JM, Berdal A, Simon S. Biodentine induces immortalized murine pulp cell differentiation into odontoblast-like cells and stimulates biomineralization. J Endod. 2012; 38:1220–1226.
Article18. Koubi S, Elmerini H, Koubi G, Tassery H, Camps J. Quantitative evaluation by glucose diffusion of microleakage in aged calcium silicate-based open-sandwich restorations. Int J Dent. 2012; 2012:105863.
Article19. Nowicka A, Lipski M, Parafiniuk M, Sporniak-Tutak K, Lichota D, Kosierkiewicz A, Kaczmarek W, Buczkowska-Radlińska J. Response of human dental pulp capped with biodentine and mineral trioxide aggregate. J Endod. 2013; 39:743–747.
Article20. Camilleri J, Sorrentino F, Damidot D. Investigation of the hydration and bioactivity of radiopacified tricalcium silicate cement, Biodentine and MTA Angelus. Dent Mater. 2013; 29:580–593.
Article21. Lee BN, Lee KN, Koh JT, Min KS, Chang HS, Hwang IN, Hwang YC, Oh WM. Effects of 3 endodontic bioactive cements on osteogenic differentiation in mesenchymal stem cells. J Endod. 2014; 40:1217–1222.
Article22. Jung JY, Woo SM, Lee BN, Kon JT, Nör JE, Hwang YC. Effect of Biodentine and Bioaggregate on odontoblastic differentiation via mitogen-activated protein kinase pathway in human dental pulp cells. Int Endod J. 2015; 48:177–184.
Article23. Zhang H, Pappen FG, Haapasalo M. Dentin enhances the antibacterial effect of mineral trioxide aggregate and bioaggregate. J Endod. 2009; 35:221–224.
Article24. Hashem AA, Wanees Amin SA. The effect of acidity on dislodgment resistance of mineral trioxide aggregate and bioaggregate in furcation perforations: an in vitro comparative study. J Endod. 2012; 38:245–249.
Article25. Grech L, Mallia B, Camilleri J. Investigation of the physical properties of tricalcium silicate cement-based root-end filling materials. Dent Mater. 2013; 29:e20–e28.
Article26. Batur YB, Acar G, Yalcin Y, Dindar S, Sancakli H, Erdemir U. The cytotoxic evaluation of mineral trioxide aggregate and bioaggregate in the subcutaneous connective tissue of rats. Med Oral Patol Oral Cir Bucal. 2013; 18:e745–e751.
Article27. Nielsen RB, Alyassin AM, Peters DD, Carnes DL, Lancaster J. Microcomputed tomography: an advanced system for detailed endodontic research. J Endod. 1995; 21:561–568.
Article28. Verma P, Love RM. A Micro CT study of the mesiobuccal root canal morphology of the maxillary first molar tooth. Int Endod J. 2011; 44:210–217.
Article29. Iwamoto CE, Adachi E, Pameijer CH, Barnes D, Romberg EE, Jefferies S. Clinical and histological evaluation of white ProRoot MTA in direct pulp capping. Am J Dent. 2006; 19:85–90.30. Aeinehchi M, Eslami B, Ghanbariha M, Saffar AS. Mineral trioxide aggregate (MTA) and calcium hydroxide as pulp-capping agents in human teeth: a preliminary report. Int Endod J. 2003; 36:225–231.
Article31. Nair PN, Duncan HF, Pitt Ford TR, Luder HU. Histological, ultrastructural and quantitative investigations on the response of healthy human pulps to experimental capping with mineral trioxide aggregate: a randomized controlled trial. Int Endod J. 2008; 41:128–150.
Article32. Koh ET, McDonald F, Pitt Ford TR, Torabinejad M. Cellular response to mineral trioxide aggregate. J Endod. 1998; 24:543–547.
Article33. Seux D, Couble ML, Hartmann DJ, Gauthier JP, Magloire H. Odontoblast-like cytodifferentiation of human dental pulp cells in vitro in the presence of a calcium hydroxide-containing cement. Arch Oral Biol. 1991; 36:117–128.
Article34. Laurent P, Camps J, About I. Biodentine(TM) induces TGF-β1 release from human pulp cells and early dental pulp mineralization. Int Endod J. 2012; 45:439–448.
Article35. Laurent P, Camps J, De Méo M, Déjou J, About I. Induction of specific cell responses to a Ca3SiO5-based posterior restorative material. Dent Mater. 2008; 24:1486–1494.
Article36. Tran XV, Gorin C, Willig C, Baroukh B, Pellat B, Decup F, Opsahl Vital S, Chaussain C, Boukpessi T. Effect of a calcium-silicate-based restorative cement on pulp repair. J Dent Res. 2012; 91:1166–1171.
Article37. Yan P, Yuan Z, Jiang H, Peng B, Bian Z. Effect of bioaggregate on differentiation of human periodontal ligament fibroblasts. Int Endod J. 2010; 43:1116–1121.
Article38. Yuan Z, Peng B, Jiang H, Bian Z, Yan P. Effect of bioaggregate on mineral-associated gene expression in osteoblast cells. J Endod. 2010; 36:1145–1148.
Article39. Zhang S, Yang X, Fan M. BioAggregate and iRoot BP Plus optimize the proliferation and mineralization ability of human dental pulp cells. Int Endod J. 2013; 46:923–929.
Article40. Saghiri MA, Tanideh N, Garcia-Godoy F, Lotfi M, Karamifar K, Amanat D. Subcutaneous connective tissue reactions to various endodontic biomaterials: an animal study. J Dent Res Dent Clin Dent Prospects. 2013; 7:15–21.41. Tziafas D, Belibasakis G, Veis A, Papadimitriou S. Dentin regeneration in vital pulp therapy: design principles. Adv Dent Res. 2001; 15:96–100.42. Min KS, Park HJ, Lee SK, Park SH, Hong CU, Kim HW, Lee HH, Kim EC. Effect of mineral trioxide aggregate on dentin bridge formation and expression of dentin sialoprotein and heme oxygenase-1 in human dental pulp. J Endod. 2008; 34:666–670.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hard tissue formation after direct pulp capping with osteostatin and MTA in vivo
- Histological Study of Reparative Dentin Formation after Direct Pulp Capping and Pulpotomy using MTA
- Effects of calcium silicate cements on neuronal conductivity
- Effect of Mineral Trioxide Aggregate and Calcium Hydroxide on Reparative Dentin Formation in Rats
- The push-out bond strength of BIOfactor mineral trioxide aggregate, a novel root repair material