Pediatr Gastroenterol Hepatol Nutr.
2015 Mar;18(1):39-47. 10.5223/pghn.2015.18.1.39.
Laboratory Markers Indicating Gastrointestinal Involvement of Henoch-Schonlein Purpura in Children
- Affiliations
-
- 1Department of Pediatrics, Kangwon National University Hospital, Kangwon National University School of Medicine, Chuncheon, Korea.
- 2Department of Pediatrics, Seoul National University Bundang Hospital, Seongnam, Korea. hryang@snubh.org
- KMID: 2315534
- DOI: http://doi.org/10.5223/pghn.2015.18.1.39
Abstract
- PURPOSE
To determine clinically useful biochemical markers reflecting disease activity and/or gastrointestinal (GI) tract involvement in Henoch-Schonlein purpura (HSP).
METHODS
A total of 185 children with HSP and 130 controls were included. Laboratory data indicating inflammation, standard coagulation, and activated coagulation were analyzed for the HSP patients, including measurements of the hemoglobin level, white blood cell (WBC) count, absolute neutrophil count (ANC), platelet count, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) level, prothrombin time, activated partial thromboplastin time, and fibrinogen, D-dimer, and fibrin degradation product (FDP) levels. The clinical scores of the skin, joints, abdomen, and kidneys were assessed during the acute and convalescence phases of HSP.
RESULTS
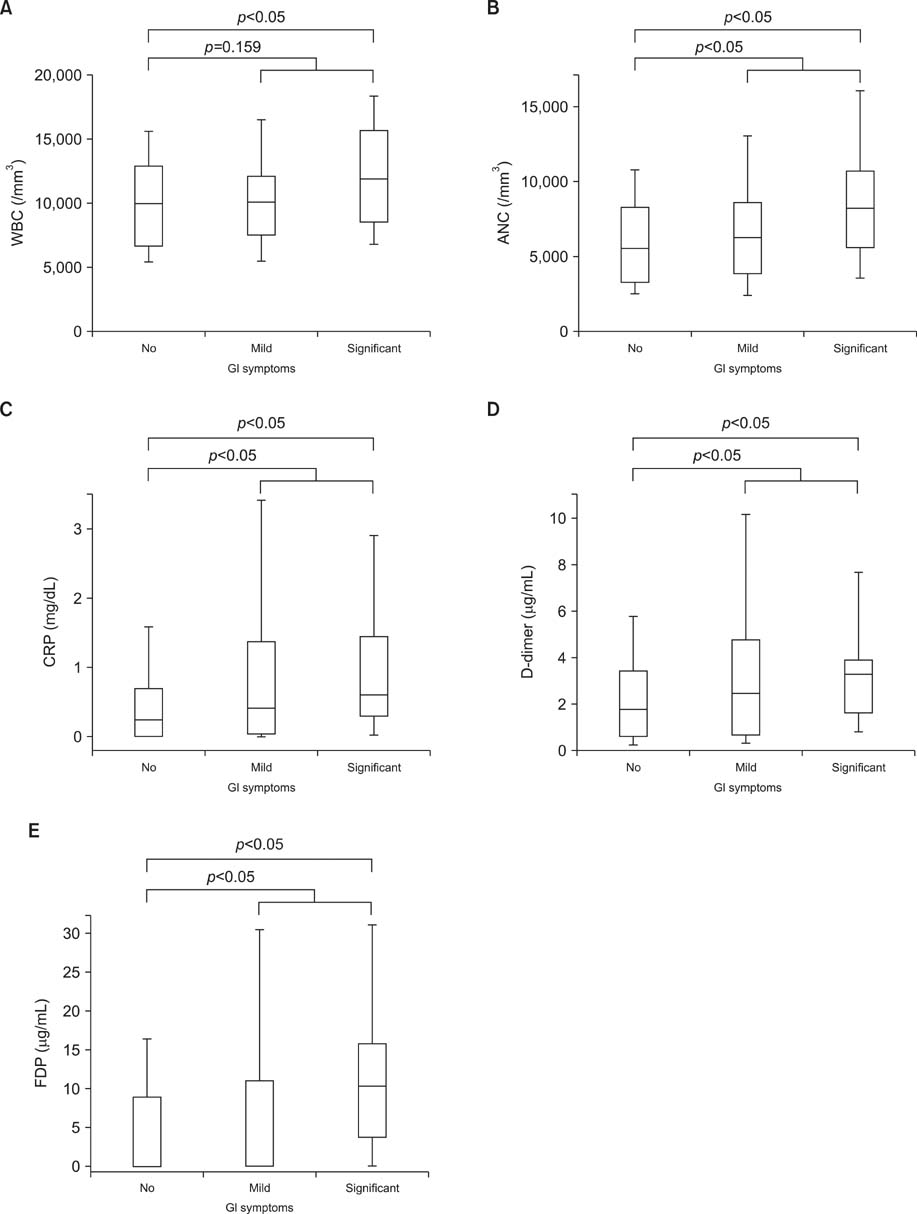
The WBC count, ANC, ESR, and CRP, fibrinogen, D-dimer, and FDP levels were significantly higher in the acute phase compared with the convalescent phase of HSP (p<0.05). The total clinical scores were more strongly correlated with the D-dimer (r=0.371, p<0.001) and FDP (r=0.369, p<0.001) levels than with inflammatory markers, such as the WBC count (r=0.241, p=0.001), ANC (r=0.261, p<0.001), and CRP (r=0.260, p<0.001) levels. The patients with GI symptoms had significantly higher ANC (median [interquartile range], 7,138.0 [4,446.4-9,470.0] vs. 5,534.1 [3,263.0-8,153.5], p<0.05) and CRP (0.49 [0.15-1.38] vs. 0.23 [0.01-0.67], p<0.05), D-dimer (2.63 [1.20-4.09] vs. 1.75 [0.62-3.39]), and FDP (7.10 [0.01-13.65] vs. 0.10 [0.01-7.90], p<0.05) levels than those without GI symptoms.
CONCLUSION
D-dimer and FDPs are more strongly associated with disease activity and more consistently reflect GI involvement than inflammatory markers during the acute phase of HSP.
Keyword
MeSH Terms
-
Abdomen
Biomarkers*
Blood Coagulation
Blood Sedimentation
C-Reactive Protein
Child*
Convalescence
Fibrin
Fibrin Fibrinogen Degradation Products
Fibrinogen
Humans
Inflammation
Joints
Kidney
Leukocytes
Neutrophils
Partial Thromboplastin Time
Platelet Count
Prothrombin Time
Purpura
Purpura, Schoenlein-Henoch*
Skin
C-Reactive Protein
Fibrin
Fibrin Fibrinogen Degradation Products
Fibrinogen
Figure
Cited by 2 articles
-
What We Know about Henoch-Schönlein Purpura in Children up to Date?
Hye Ran Yang
J Korean Med Sci. 2018;33(25):. doi: 10.3346/jkms.2018.33.e199.Henoch-Schonlein Purpura in Children Hospitalized at a Tertiary Hospital during 2004-2015 in Korea: Epidemiology and Clinical Management
Yong Hee Lee, Yu Bin Kim, Ja Wook Koo, Ju-Young Chung
Pediatr Gastroenterol Hepatol Nutr. 2016;19(3):175-185. doi: 10.5223/pghn.2016.19.3.175.
Reference
-
1. Mills JA, Michel BA, Bloch DA, Calabrese LH, Hunder GG, Arend WP, et al. The American College of Rheumatology 1990 criteria for the classification of Henoch-Schönlein purpura. Arthritis Rheum. 1990; 33:1114–1121.
Article2. Ozen S, Ruperto N, Dillon MJ, Bagga A, Barron K, Davin JC, et al. EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann Rheum Dis. 2006; 65:936–941.
Article3. Kim S, Yoon J, Jeong S. Comparison of the clinical manifestations and prognosis of Henoch-Schonlein purpura in children with and without abdominal pain. Korean J Pediatr Gastroenterol Nutr. 2011; 14:359–367.
Article4. Trapani S, Micheli A, Grisolia F, Resti M, Chiappini E, Falcini F, et al. Henoch Schonlein purpura in childhood: epidemiological and clinical analysis of 150 cases over a 5-year period and review of literature. Semin Arthritis Rheum. 2005; 35:143–153.
Article5. Calviño MC, Llorca J, García-Porrúa C, Fernández-Iglesias JL, Rodriguez-Ledo P, González-Gay MA. Henoch-Schönlein purpura in children from northwestern Spain: a 20-year epidemiologic and clinical study. Medicine (Baltimore). 2001; 80:279–290.6. Hung SP, Yang YH, Lin YT, Wang LC, Lee JH, Chiang BL. Clinical manifestations and outcomes of Henoch-Schönlein purpura: comparison between adults and children. Pediatr Neonatol. 2009; 50:162–168.
Article7. Martinez-Frontanilla LA, Haase GM, Ernster JA, Bailey WC. Surgical complications in Henoch-Schönlein Purpura. J Pediatr Surg. 1984; 19:434–436.
Article8. Faille-Kuyber EH, Kater L, Kooiker CJ, Dorhout Mees EJ. IgA-deposits in cutaneous blood-vessel walls and mesangium in Henoch-Schönlein syndrome. Lancet. 1973; 1:892–893.9. Besbas N, Saatci U, Ruacan S, Ozen S, Sungur A, Bakkaloglu A, et al. The role of cytokines in Henoch Schonlein purpura. Scand J Rheumatol. 1997; 26:456–460.
Article10. Claudy A. Coagulation and fibrinolysis in cutaneous vasculitis. Clin Dermatol. 1999; 17:615–618.
Article11. Ateş E, Bakkaloğlu A, Saatçi U, Söylemezoğlu O. von Willebrand factor antigen compared with other factors in vasculitic syndromes. Arch Dis Child. 1994; 70:40–43.
Article12. Hergesell O, Andrassy K, Nawroth P. Elevated levels of markers of endothelial cell damage and markers of activated coagulation in patients with systemic necrotizing vasculitis. Thromb Haemost. 1996; 75:892–898.
Article13. Rose PE, Struthers GS, Robertson M, Kavi J, Chant I, Taylor CM. Factor VIII von Willebrand protein in haemolytic uraemic syndrome and systemic vasculitides. Lancet. 1990; 335:500–502.
Article14. De Mattia D, Penza R, Giordano P, Del Vecchio GC, Aceto G, Altomare M, et al. von Willebrand factor and factor XIII in children with Henoch-Schonlein purpura. Pediatr Nephrol. 1995; 9:603–605.
Article15. Yilmaz D, Kavakli K, Ozkayin N. The elevated markers of hypercoagulability in children with Henoch-Schönlein purpura. Pediatr Hematol Oncol. 2005; 22:41–48.
Article16. Saulsbury FT. Henoch-Schönlein purpura. Curr Opin Rheumatol. 2010; 22:598–602.
Article17. Yang YH, Chuang YH, Wang LC, Huang HY, Gershwin ME, Chiang BL. The immunobiology of Henoch-Schönlein purpura. Autoimmun Rev. 2008; 7:179–184.
Article18. Yang YH, Yu HH, Chiang BL. The diagnosis and classification of Henoch-Schönlein purpura: an updated review. Autoimmun Rev. 2014; 13:355–358.
Article19. Levi M, van der Poll T. Two-way interactions between inflammation and coagulation. Trends Cardiovasc Med. 2005; 15:254–259.
Article20. Szaba FM, Smiley ST. Roles for thrombin and fibrin( ogen) in cytokine/chemokine production and macrophage adhesion in vivo. Blood. 2002; 99:1053–1059.
Article21. Del Vecchio GC, Penza R, Altomare M, Piacente L, Aceto G, Lassandro G, et al. Cytokine pattern and endothelium damage markers in Henoch-Schönlein purpura. Immunopharmacol Immunotoxicol. 2008; 30:623–629.
Article22. Dalens B, Travade P, Labbé A, Bezou MJ. Diagnostic and prognostic value of fibrin stabilising factor in Schönlein-Henoch syndrome. Arch Dis Child. 1983; 58:12–14.
Article23. Kamitsuji H, Tani K, Yasui M, Taniguchi A, Taira K, Tsukada S, et al. Activity of blood coagulation factor XIII as a prognostic indicator in patients with Henoch-Schönlein purpura. Efficacy of factor XIII substitution. Eur J Pediatr. 1987; 146:519–523.
Article24. Brendel-Müller K, Hahn A, Schneppenheim R, Santer R. Laboratory signs of activated coagulation are common in Henoch-Schönlein purpura. Pediatr Nephrol. 2001; 16:1084–1088.
Article25. Kang Y, Park JS, Ha YJ, Kang MI, Park HJ, Lee SW, et al. Differences in clinical manifestations and outcomes between adult and child patients with Henoch-Schönlein purpura. J Korean Med Sci. 2014; 29:198–203.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Henoch-Schonlein Purpura Involving Colon Mimicking Colon Cancer
- Intramural Hematoma of the Duodenum following Endoscopic Biopsy in a Child with Henoch-Schonlein Purpura
- A Case of Henoch-Schonlein Purpura Complicated by Hemorrhagic Ascites and Multiple Upper Gastrointestinal Bleeding
- A Case of Henoch - Schoenlein Purpura Involving G-I Tract
- Endoscopic Findings of Children with Henoch-Schonlein Purpura