Korean J Urol.
2011 Sep;52(9):632-636.
Changes in Sexual Function in Benign Prostatic Hyperplasia Patients Taking Dutasteride: 1-Year Follow-Up Results
- Affiliations
-
- 1Department of Urology, Chung-Ang University College of Medicine, Seoul, Korea. saeckim@unitel.co.kr
Abstract
- PURPOSE
Sexual adverse events (AEs), a major cause for discontinuing 5alpha-reductase inhibitor (5ARI) therapy for benign prostatic hyperplasia (BPH), are known to occur most frequently early in therapy and appear to decline over time. The aim of this study was to investigate the changes in sexual function occurring with dutasteride treatment during a 1-year follow-up period in Korean men.
MATERIALS AND METHODS
Using the International Index of Erectile Function, we prospectively evaluated, after 1, 3, 6, 9, and 12 months of treatment, the changes in sexual function of 55 outpatients (mean age 62.3+/-7.2 years) with BPH (mean volume 48.9+/-16.0 g) who had relatively good erectile function (EF) and were treated with dutasteride for at least 1 year.
RESULTS
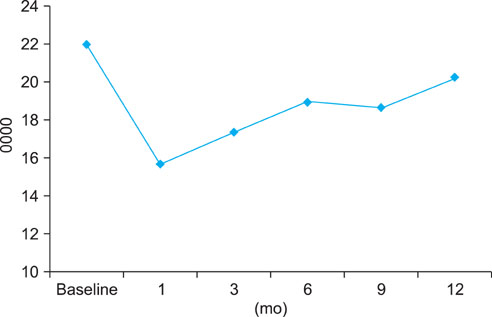
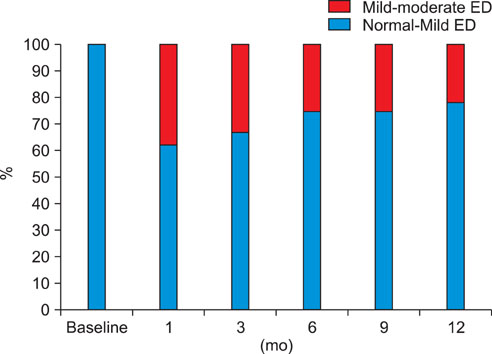
EF scores showed the most significant decrease at 1 month (p<0.01). Function gradually recovered thereafter but was still significantly decreased after 12 months of treatment (p<0.05). The scores for orgasmic function and sexual desire also showed the most significant reduction at 1 month but were restored to the baseline level at 6 months. No significant correlation was observed between changes in sexual function and prostate-specific antigen level, prostate volume, or International Prostate Symptom Scores.
CONCLUSIONS
After 1 month of treatment, dutasteride therapy resulted in a significant reduction in all investigated sexual functions. Overall, recovery in sexual function was noted at 3 months, and orgasmic function and sexual desire were restored to baseline levels at 6 months. However, EF was still significantly reduced at 12 months.
MeSH Terms
Figure
Reference
-
1. Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998. 97:1837–1847.2. Chung BH, Lee JY, Lee SH, Yoo SJ, Lee SW, Oh CY. Safety and efficacy of the simultaneous administration of udenafil and an alpha-blocker in men with erectile dysfunction concomitant with BPH/LUTS. Int J Impot Res. 2009. 21:122–128.3. Nickel JC, Elhilali M, Emberton M, Vallancien G. Alf-One Study Group. The beneficial effect of alfuzosin 10 mg once daily in 'real-life' practice on lower urinary tract symptoms (LUTS), quality of life and sexual dysfunction in men with LUTS and painful ejaculation. BJU Int. 2006. 97:1242–1246.4. Iehlé C, Délos S, Guirou O, Tate R, Raynaud JP, Martin PM. Human prostatic steroid 5 alpha-reductase isoforms--a comparative study of selective inhibitors. J Steroid Biochem Mol Biol. 1995. 54:273–279.5. Marihart S, Harik M, Djavan B. Dutasteride: a review of current data on a novel dual inhibitor of 5alpha reductase. Rev Urol. 2005. 7:203–210.6. Edwards JE, Moore RA. Finasteride in the treatment of clinical benign prostatic hyperplasia: a systematic review of randomised trials. BMC Urol. 2002. 2:14.7. Erdemir F, Harbin A, Hellstrom WJ. 5-alpha reductase inhibitors and erectile dysfunction: the connection. J Sex Med. 2008. 5:2917–2924.8. Wessells H, Roy J, Bannow J, Grayhack J, Matsumoto AM, Tenover L, et al. Incidence and severity of sexual adverse experiences in finasteride and placebo-treated men with benign prostatic hyperplasia. Urology. 2003. 61:579–584.9. Stoner E. Three-year safety and efficacy data on the use of finasteride in the treatment of benign prostatic hyperplasia. Urology. 1994. 43:284–292.10. Roehrborn CG, Marks LS, Fenter T, Freedman S, Tuttle J, Gittleman M, et al. Efficacy and safety of dutasteride in the four-year treatment of men with benign prostatic hyperplasia. Urology. 2004. 63:709–715.11. Gormley GJ, Stoner E, Bruskewitz RC, Imperato-McGinley J, Walsh PC, McConnell JD, et al. The effect of finasteride in men with benign prostatic hyperplasia. 1992. J Urol. 2002. 167:1102–1107.12. Sawaya ME, Price VH. Different levels of 5alpha-reductase type I and II, aromatase, and androgen receptor in hair follicles of women and men with androgenetic alopecia. J Invest Dermatol. 1997. 109:296–300.13. Bartsch G, Rittmaster RS, Klocker H. Dihydrotestosterone and the concept of 5alpha-reductase inhibition in human benign prostatic hyperplasia. Eur Urol. 2000. 37:367–380.14. AUA Practice Guidelines Committee. AUA guideline on management of benign prostatic hyperplasia (2003). Chapter 1: Diagnosis and treatment recommendations. J Urol. 2003. 170:530–547.15. Uygur MC, Arik AI, Altuğ U, Erol D. Effects of the 5 alpha-reductase inhibitor finasteride on serum levels of gonadal, adrenal, and hypophyseal hormones and its clinical significance: a prospective clinical study. Steroids. 1998. 63:208–213.16. Andriole GL, Kirby R. Safety and tolerability of the dual 5alpha-reductase inhibitor dutasteride in the treatment of benign prostatic hyperplasia. Eur Urol. 2003. 44:82–88.17. Park KH, Kim SW, Kim KD, Paick JS. Effects of androgens on the expression of nitric oxide synthase mRNAs in rat corpus cavernosum. BJU Int. 1999. 83:327–333.18. Seo SI, Kim SW, Paick JS. The effects of androgen on penile reflex, erectile response to electrical stimulation and penile NOS activity in the rat. Asian J Androl. 1999. 1:169–174.19. Mondaini N, Gontero P, Giubilei G, Lombardi G, Cai T, Gavazzi A, et al. Finasteride 5 mg and sexual side effects: how many of these are related to a nocebo phenomenon? J Sex Med. 2007. 4:1708–1712.20. Riley AJ. Life-long absence of sexual drive in a woman associated with 5-dihydrotestosterone deficiency. J Sex Marital Ther. 1999. 25:73–78.21. Cai LQ, Fratianni CM, Gautier T, Imperato-McGinley J. Dihydrotestosterone regulation of semen in male pseudohermaphrodites with 5 alpha-reductase-2 deficiency. J Clin Endocrinol Metab. 1994. 79:409–414.22. Guess HA, Gormley GJ, Stoner E, Oesterling JE. The effect of finasteride on prostate specific antigen: review of available data. J Urol. 1996. 155:3–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Different Reduction Rate of Prostate-Specific Antigen in Dutasteride and Finasteride
- Positive Role of Dutasteride in the Treatment of Localized Prostate Cancer
- Effects of Doxazosin on Sexual Function in Patients with Benign Prostatic Hyperplasia
- Benign Prostatic Hyperplasia and Sexual Dysfunction
- The Effect of Short Term Dutasteride Therapy on Microvessel Density in Benign Prostatic Hyperplasia