Nutr Res Pract.
2012 Aug;6(4):294-300.
Effect of resveratrol on the metastasis of 4T1 mouse breast cancer cells in vitro and in vivo
- Affiliations
-
- 1Department of Food Science and Nutrition, Dongseo University, Busan 617-716, Korea.
- 2Department of Food Science and Nutrition, Dankook University, 126, Jukjeon-dong, Suji-gu, Yongin-si, Gyunggi 448-701, Korea. wkkim@dankook.ac.kr
Abstract
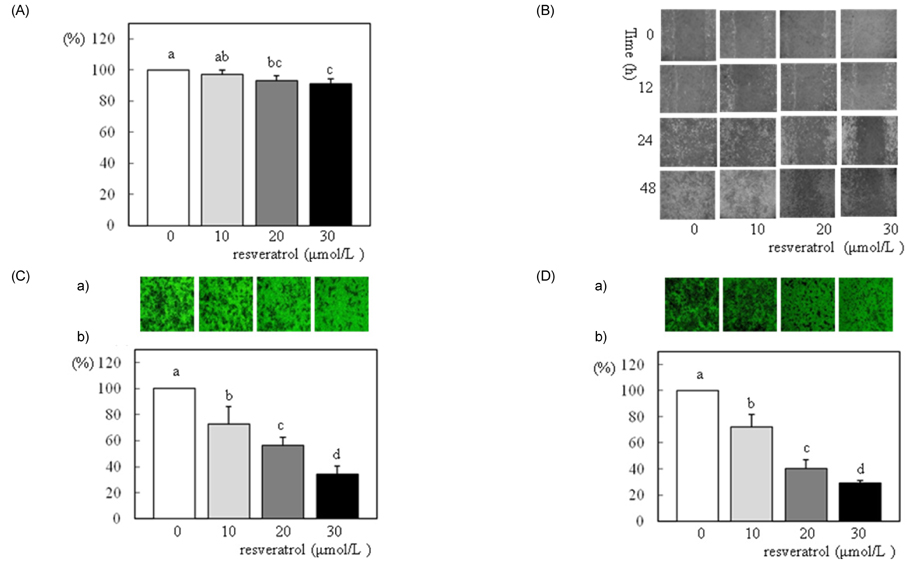
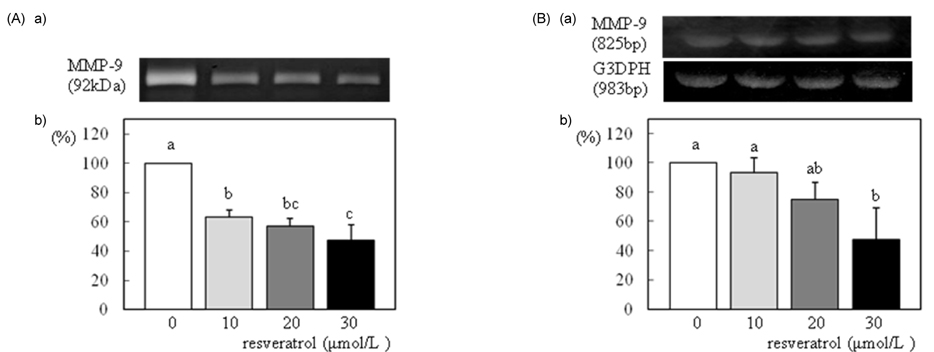
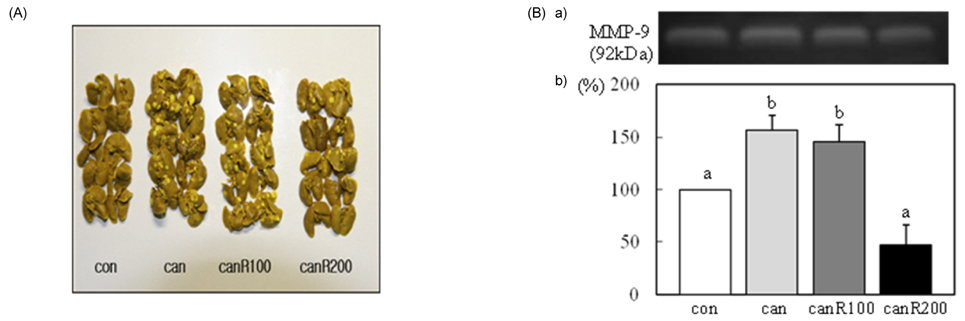
- We investigated the effects of resveratrol on metastasis in in vitro and in vivo systems. 4T1 cells were cultured in the presence of various concentrations (0-30 micromol/L) of resveratrol. For experimental metastasis, BALB/c mice were injected intravenously with 4T1 cells in the tail vein, and were orally administered various concentrations (0, 100, or 200 mg/kg Body weight) of resveratrol for 21 days. After resveratrol treatment, cell adhesion, wound migration, invasion, and MMP-9 activity were significantly decreased in a dose-dependent manner in 4T1 cells (P < 0.05). The numbers of pulmonary nodules were significantly decreased in mice fed the resveratrol (P < 0.05). The plasma MMP-9 activity was decreased in response to treatment with resveratrol in mice (P < 0.05). We conclude that resveratrol inhibits cancer metastasis both in vitro and in vivo, and this inhibition is likely due to the decrease in MMP-9 activity caused by resveratrol.
MeSH Terms
Figure
Reference
-
1. Zhou HB, Yan Y, Sun YN, Zhu JR. Resveratrol induces apoptosis in human esophageal carcinoma cells. World J Gastroenterol. 2003. 9:408–411.
Article2. Kopp P. Resveratrol, a phytoestrogen found in red wine. A possible explanation for the conundrum of the 'French paradox'? Eur J Endocrinol. 1998. 138:619–620.
Article3. Jang M, Pezzuto JM. Cancer chemopreventive activity of resveratrol. Drugs Exp Clin Res. 1999. 25:65–77.4. Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997. 275:218–220.
Article5. Frankel EN, Waterhouse AL, Kinsella JE. Inhibition of human LDL oxidation by resveratrol. Lancet. 1993. 341:1103–1104.
Article6. Fauconneau B, Waffo-Teguo P, Huguet F, Barrier L, Decendit A, Merillon JM. Comparative study of radical scavenger and antioxidant properties of phenolic compounds from Vitis vinifera cell cultures using in vitro tests. Life Sci. 1997. 61:2103–2110.
Article7. Pace-Asciak CR, Rounova O, Hahn SE, Diamandis EP, Goldberg DM. Wines and grape juices as modulators of platelet aggregation in healthy human subjects. Clin Chim Acta. 1996. 246:163–182.
Article8. Liu BL, Zhang X, Zhang W, Zhen HN. New enlightenment of French Paradox: resveratrol's potential for cancer chemoprevention and anti-cancer therapy. Cancer Biol Ther. 2007. 6:1833–1836.
Article9. Sun AY, Simonyi A, Sun GY. The "French Paradox" and beyond: neuroprotective effects of polyphenols. Free Radic Biol Med. 2002. 32:314–318.10. Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res. 2004. 24:2783–2840.11. Athar M, Back JH, Tang X, Kim KH, Kopelovich L, Bickers DR, Kim AL. Resveratrol: a review of preclinical studies for human cancer prevention. Toxicol Appl Pharmacol. 2007. 224:274–283.
Article12. Yoon SO, Kim MM, Chung AS. Inhibitory effect of selenite on invasion of HT1080 tumor cells. J Biol Chem. 2001. 276:20085–20092.
Article13. Mook OR, Frederiks WM, Van Noorden CJ. The role of gelatinases in colorectal cancer progression and metastasis. Biochim Biophys Acta. 2004. 1705:69–89.
Article14. Srivastava KC. Aqueous extracts of onion, garlic and ginger inhibit platelet aggregation and alter arachidonic acid metabolism. Biomed Biochim Acta. 1984. 43:S335–S346.15. Freije JM, Balbín M, Pendás AM, Sánchez LM, Puente XS, López-Otín C. Matrix metalloproteinases and tumor progression. Adv Exp Med Biol. 2003. 532:91–107.
Article16. Kim JE, Kim HS, Shin YJ, Lee CS, Won C, Lee SA, Lee JW, Kim Y, Kang JS, Ye SK, Chung MH. LYR71, a derivative of trimeric resveratrol, inhibits tumorigenesis by blocking STAT3-mediated matrix metalloproteinase 9 expression. Exp Mol Med. 2008. 40:514–522.
Article17. Tao K, Fang M, Alroy J, Sahagian GG. Imagable 4T1 model for the study of late stage breast cancer. BMC Cancer. 2008. 8:228.
Article18. Lee HS, Seo EY, Kang NE, Kim WK. [6]-Gingerol inhibits metastasis of MDA-MB-231 human breast cancer cells. J Nutr Biochem. 2008. 19:313–319.
Article19. Soel SM, Choi OS, Bang MH, Yoon Park JH, Kim WK. Influence of conjugated linoleic acid isomers on the metastasis of colon cancer cells in vitro and in vivo. J Nutr Biochem. 2007. 18:650–657.
Article20. Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993. 123:1939–1951.
Article21. Pignatelli M, Stamp G. Integrins in tumour development and spread. Cancer Surv. 1995. 24:113–127.22. Stetler-Stevenson WG, Aznavoorian S, Liotta LA. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol. 1993. 9:541–573.
Article23. Yu AE, Hewitt RE, Kleiner DE, Stetler-Stevenson WG. Molecular regulation of cellular invasion--role of gelatinase A and TIMP-2. Biochem Cell Biol. 1996. 74:823–831.
Article24. Liotta LA, Tryggvason K, Garbisa S, Hart I, Foltz CM, Shafie S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature. 1980. 284:67–68.
Article25. Chen J, Thompson LU. Lignans and tamoxifen, alone or in combination, reduce human breast cancer cell adhesion, invasion and migration in vitro. Breast Cancer Res Treat. 2003. 80:163–170.
Article26. Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001. 17:463–516.
Article27. Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002. 2:161–174.
Article28. Scorilas A, Karameris A, Arnogiannaki N, Ardavanis A, Bassilopoulos P, Trangas T, Talieri M. Overexpression of matrix-metalloproteinase-9 in human breast cancer: a potential favourable indicator in node-negative patients. Br J Cancer. 2001. 84:1488–1496.
Article29. Tang FY, Chiang EP, Sun YC. Resveratrol inhibits heregulinbeta1-mediated matrix metalloproteinase-9 expression and cell invasion in human breast cancer cells. J Nutr Biochem. 2008. 19:287–294.
Article30. Pan MH, Chiou YS, Chen WJ, Wang JM, Badmaev V, Ho CT. Pterostilbene inhibited tumor invasion via suppressing multiple signal transduction pathways in human hepatocellular carcinoma cells. Carcinogenesis. 2009. 30:1234–1242.
Article31. Banerjee S, Bueso-Ramos C, Aggarwal BB. Suppression of 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in rats by resveratrol: role of nuclear factor-kappaB, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Res. 2002. 62:4945–4954.32. Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007. 7:41–51.
Article33. Manna SK, Mukhopadhyay A, Aggarwal BB. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol. 2000. 164:6509–6519.
Article34. Birrell MA, McCluskie K, Wong S, Donnelly LE, Barnes PJ, Belvisi MG. Resveratrol, an extract of red wine, inhibits lipopolysaccharide induced airway neutrophilia and inflammatory mediators through an NF-kappaB-independent mechanism. FASEB J. 2005. 19:840–841.35. Kowalski J, Samojedny A, Paul M, Pietsz G, Wilczok T. Effect of apigenin, kaempferol and resveratrol on the expression of interleukin-1beta and tumor necrosis factor-alpha genes in J774.2 macrophages. Pharmacol Rep. 2005. 57:390–394.36. Bove K, Lincoln DW, Tsan MF. Effect of resveratrol on growth of 4T1 breast cancer cells in vitro and in vivo. Biochem Biophys Res Commun. 2002. 291:1001–1005.
Article37. Carbó N, Costelli P, Baccino FM, López-Soriano FJ, Argilés JM. Resveratrol, a natural product present in wine, decreases tumour growth in a rat tumour model. Biochem Biophys Res Commun. 1999. 254:739–743.
Article38. Kimura Y, Okuda H. Resveratrol isolated from Polygonum cuspidatum root prevents tumor growth and metastasis to lung and tumor-induced neovascularization in Lewis lung carcinomabearing mice. J Nutr. 2001. 131:1844–1849.
Article39. Weng YL, Liao HF, Li AF, Chang JC, Chiou RY. Oral administration of resveratrol in suppression of pulmonary metastasis of BALB/c mice challenged with CT26 colorectal adenocarcinoma cells. Mol Nutr Food Res. 2010. 54:259–267.
Article40. Busquets S, Ametller E, Fuster G, Olivan M, Raab V, Argilés JM, López-Soriano FJ. Resveratrol, a natural diphenol, reduces metastatic growth in an experimental cancer model. Cancer Lett. 2007. 245:144–148.
Article41. Bhat KP, Lantvit D, Christov K, Mehta RG, Moon RC, Pezzuto JM. Estrogenic and antiestrogenic properties of resveratrol in mammary tumor models. Cancer Res. 2001. 61:7456–7463.42. Li D, Chen X, Yu H. Resveratrol inhibits MMP-2 expression of hepatoma in nude mice. J Anim Vet Adv. 2011. 10:33–37.
Article43. Chen Y, Tseng SH, Lai HS, Chen WJ. Resveratrol-induced cellular apoptosis and cell cycle arrest in neuroblastoma cells and antitumor effects on neuroblastoma in mice. Surgery. 2004. 136:57–66.
Article44. Tseng SH, Lin SM, Chen JC, Su YH, Huang HY, Chen CK, Lin PY, Chen Y. Resveratrol suppresses the angiogenesis and tumor growth of gliomas in rats. Clin Cancer Res. 2004. 10:2190–2202.
Article45. Harper CE, Patel BB, Wang J, Arabshahi A, Eltoum IA, Lamartiniere CA. Resveratrol suppresses prostate cancer progression in transgenic mice. Carcinogenesis. 2007. 28:1946–1953.
Article46. Weng CJ, Yang YT, Ho CT, Yen GC. Mechanisms of apoptotic effects induced by resveratrol, dibenzoylmethane, and their analogues on human lung carcinoma cells. J Agric Food Chem. 2009. 57:5235–5243.
Article47. Liu PL, Tsai JR, Charles AL, Hwang JJ, Chou SH, Ping YH, Lin FY, Chen YL, Hung CY, Chen WC, Chen YH, Chong IW. Resveratrol inhibits human lung adenocarcinoma cell metastasis by suppressing heme oxygenase 1-mediated nuclear factorkappaB pathway and subsequently downregulating expression of matrix metalloproteinases. Mol Nutr Food Res. 2010. 54:Suppl 2. S196–S204.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- OCT4 Expression Enhances Features of Cancer Stem Cells in a Mouse Model of Breast Cancer
- Peanut sprout tea extract inhibits lung metastasis of 4T1 murine mammary carcinoma cells by suppressing the crosstalk between cancer cells and macrophages in BALB/c mice
- α-Tocopheryl Succinate Inhibits Osteolytic Bone Metastasis of Breast Cancer by Suppressing Migration of Cancer Cells and Receptor Activator of Nuclear Factor-κB Ligand Expression of Osteoblasts
- NDRG2 Expression Decreases Tumor-Induced Osteoclast Differentiation by Down-regulating ICAM1 in Breast Cancer Cells
- Dimethyl Sulfoxide Suppresses Mouse 4T1 Breast Cancer Growth by Modulating Tumor-Associated Macrophage Differentiation