Nutr Res Pract.
2011 Aug;5(4):281-287.
Antioxidant activity and analysis of proanthocyanidins from pine (Pinus densiflora) needles
- Affiliations
-
- 1Department of Food and Nutrition, Silla University, 140 Baegyang-daero 700beon-gil, Sasang-gu, Busan 617-736, Korea. mihkim@silla.ac.kr
- 2Department of Bioscience and Biotechnology, Silla University, Busan 617-736, Korea.
- 3Bioport Korea Co., Marine Bio-industry Development Center, Busan 619-912, Korea.
Abstract
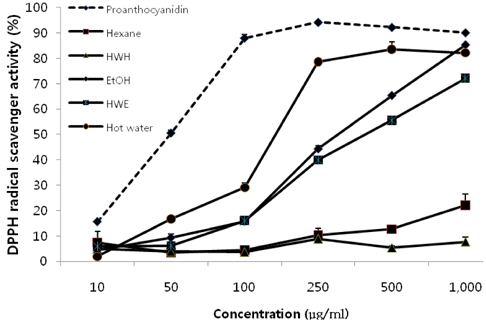
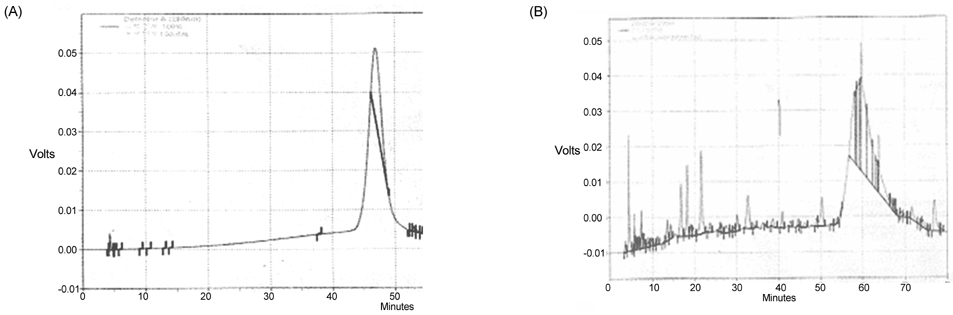
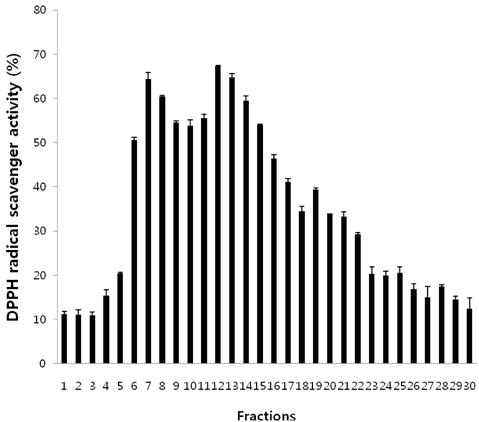
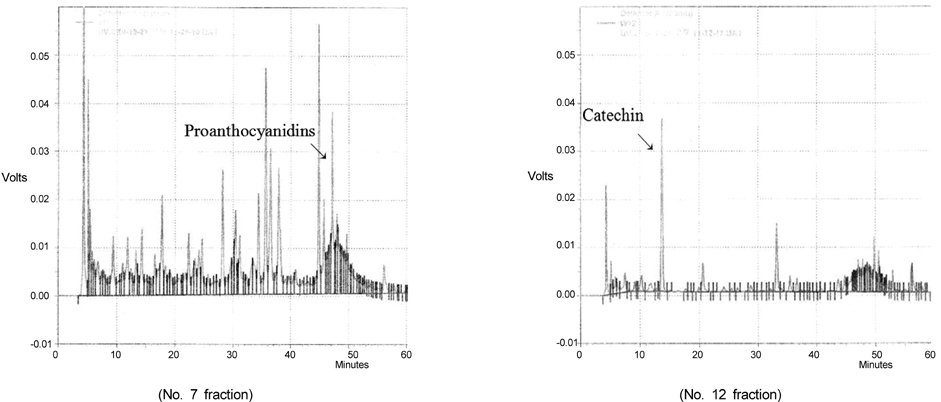
- In this study, we evaluated the antioxidant activity of pine needle extracts prepared with hot water, ethanol, hexane, hot water-hexane (HWH), and hot water-ethanol (HWE), using the DPPH (1,1-diphenyl-2-picrylhydrazyl) radical method. The hot water extract possessed superior antioxidant activity than the other extracts. We also compared the antioxidant activity of pine needle extracts through ROS inhibition activity in a cellular system using MC3T3 E-1 cells. The hot water extract exhibited the lowest ROS production. The pattern of HPLC analysis of each extract indicated that the hot water extract contained the highest proanthocyanidin level. The pine needle hot-water extract was then isolated and fractionated with Sephadex LH-20 column chromatography to determine the major contributor to its antioxidant activity. The No.7 and 12 fractions had high antioxidant activities, that is, the highest contents of proanthocyanidins and catechins, respectively. These results indicate that the antioxidant activity of procyanidins from the hot water extract of pine needles is positively related to not only polymeric proanthocyanidins but also to monomeric catechins. Moreover, the antioxidant activity of the pine needle hot water extract was similar to well-known antioxidants, such as vitamin C. This suggests that pine needle proanthocyanidins and catechins might be of interest for use as alternative antioxidants.
Keyword
MeSH Terms
Figure
Reference
-
1. Zacchini M, de Agazio M. Spread of oxidative damage and antioxidative response through cell layers of tobacco callus after UV-C treatment. Plant Physiol Biochem. 2004. 42:445–450.
Article2. Halliwell B. Antioxidant defence mechanisms: from the beginning to the end (of the beginning). Free Radic Res. 1999. 31:261–272.
Article3. Cook NC, Samman S. Flavonoids-Chemistry, metabolism, cardioprotective effects, and dietary sources. J Nutr Biochem. 1996. 7:66–76.
Article4. Wang H, Gao XD, Zhou GC, Cai L, Yao WB. In vitro and in vivo antioxidant activity of aqueous extract from Choerospondias axillaris fruit. Food Chem. 2008. 106:888–895.5. Kim KY, Chung HJ. Flavor compounds of pine sprout tea and pine needle tea. J Agric Food Chem. 2000. 48:1269–1272.
Article6. Hsu B, Coupar IM, Na K. Antioxidant activity of hot water extract from the fruit of the Doum Palm Hyphaene thebaica. Food Chem. 2006. 98:317–328.
Article7. Jeong JB, Seo EW, Jeong HJ. Effect of extracts from pine needle against oxidative DNA damage and apoptosis induced by hydroxyl radical via antioxidant activity. Food Chem Toxicol. 2009. 47:2135–2141.
Article8. Rohdewald P. A review of the French maritime pine bark extract (Pycnogenol), a herbal medication with a diverse clinical pharmacology. Int J Clin Pharmacol Ther. 2002. 40:158–168.
Article9. Schäfer A, Chovanová Z, Muchová J, Sumegová K, Liptáková A, Duracková Z, Högger P. Inhibition of COX-1 and COX-2 activity by plasma of human volunteers after ingestion of French maritime pine bark extract (Pycnogenol). Biomed Pharmacother. 2005. 60:5–9.
Article10. Touriño S, Selga A, Jiménez A, Juliá L, Lozano C, Lizárraga D, Cascante M, Torres JL. Procyanidin fractions from pine (Pinus pinaster) bark: radical scavenging power in solution, antioxidant activity in emulsion, and antiproliferative effect in melanoma cells. J Agric Food Chem. 2005. 53:4728–4735.
Article11. Ferreira D, Slade D. Oligomeric proanthocyanidins: naturally occurring O-heterocycles. Nat Prod Rep. 2002. 19:517–541.
Article12. De Bruyne T, Pieters L, Deelstra H, Vlietinck A. Condensed vegetable tannins: biodiversity in structure and biological activities. Biochem Syst Ecol. 1999. 27:445–459.
Article13. Ariga T, Hamano M. Radical scavenging action and its mode in procyanidins B-1 and B-3 from Azuki beans to peroxyl radicals. Agric Biol Chem. 1990. 54:2499–2504.
Article14. Ricardo da Silva JM, Darman N, Fernandez Y, Mitjavila S. Oxygen free radical scavenger capacity in aqueous models of different procyanidins from grape seeds. J Agric Food Chem. 1991. 39:1549–1552.
Article15. Bagchi D, Ray SD, Patel D, Bagchi M. Protection against drug- and chemical-induced multiorgan toxicity by a novel IH636 grape seed proanthocyanidin extract. Drugs Exp Clin Res. 2001. 27:3–15.16. Sharma G, Tyagi AK, Singh RP, Chan DC, Agarwal R. Synergistic anti-cancer effects of grape seed extract and conventional cytotoxic agent doxorubicin against human breast carcinoma cells. Breast Cancer Res Treat. 2004. 85:1–12.
Article17. Porter LJ. Harborne JB, editor. The Flavonoids. Advances in Research Since 1986. 1993. London: Chapman & Hall;23–55.18. Quarles LD, Yohay DA, Lever LW, Caton R, Wenstrup RJ. Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: an in vitro model of osteoblast development. J Bone Miner Res. 1992. 7:683–692.
Article19. Kim NY, Jang MK, Lee DG, Yu KH, Jang H, Kim M, Kim SG, Yoo BH, Lee SH. Comparison of methods for proanthocyanidin extraction from pine (Pinus densiflora) needles and biological activities of the extracts. Nutr Res Pract. 2010. 4:16–22.
Article20. Duan XJ, Zhang WW, Li XM, Wang BG. Evaluation of antioxidant property of extract and fractions obtained from a red alga. Polysiphonia urceolata. Food Chem. 2006. 95:37–43.
Article21. Shon MY, Kim TH, Sung NJ. Antioxidants and free radical scavenging activity of Phellinus baunii (Phellinus of Hymenochaetoceae) extracts. Food Chem. 2003. 82:593–597.
Article22. Wong SP, Leong LP, Koh JHW. Antioxidant activities of aqueous extracts of selected plants. Food Chem. 2006. 99:775–783.
Article23. Gabetta B, Fuzzati N, Griffini A, Lolla E, Pace R, Ruffilli T, Peterlongo F. Characterization of proanthocyanidins from grape seeds. Fitoterapia. 2000. 71:162–175.
Article24. Liu X, Dong M, Chen X, Jiang M, Lv X, Yan G. Antioxidant activity and phenolics of an endophytic Xylaria sp. from Ginkgo biloba. Food Chem. 2007. 105:548–554.
Article25. Karonen M, Loponen J, Ossipov V, Pihlaja K. Analysis of procyanidins in pine bark with reversed-phase and normal-phase high-performance liquid chromatography-electrospray ionization mass spectrometry. Analytica Chimica Acta. 2004. 522:105–112.
Article26. Cho KJ, Yun CH, Yoon DY, Cho YS, Rimbach G, Packer L, Chung AS. Effect of bioflavonoids extracted from the bark of Pinus maritima on proinflammatory cytokine interleukin-1 production in lipopolysaccharide-stimulated RAW 264.7. Toxicol Appl Pharmacol. 2000. 168:64–71.
Article27. Halliwell B, Gutteridge JMC. Halliwell B, Gutteridge JMC, editors. Antioxidant defences. Free Radicals in Biology and Medicine. 1999. New York: Oxford University Press;200–216.28. Zorov DB, Juhaszova M. Sollott SJ. Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta. 2006. 1757:509–517.29. Ku CS, Sathishkumar M, Mun SP. Binding affinity of proanthocyanidin from waste Pinus radiata bark onto proline-rich bovine achilles tendon collagen type I. Chemosphere. 2007. 67:1618–1627.
Article30. Souque JM, Cheynier V, Brossaud F, Moutounet M. Polymeric proanthocyanidins from grape skins. Phytochemistry. 1996. 43:509–512.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Ectomycorrhizal Fungi on Growth of Seedlings of Pinus densiflora
- Comparison of methods for proanthocyanidin extraction from pine (Pinus densiflora) needles and biological activities of the extracts
- Ectomycorrhizal Fungal Communities of Red Pine (Pinus densiflora) Seedlings in Disturbed Sites and Undisturbed Old Forest Sites
- Two Species of Endophytic Cladosporium in Pine Trees in Korea
- Fungi Colonizing Sapwood of Japanese Red Pine Logs in Storage