Nutr Res Pract.
2011 Jun;5(3):214-218.
High glucose diets shorten lifespan of Caenorhabditis elegans via ectopic apoptosis induction
- Affiliations
-
- 1Department of Food and Nutrition, College of Natural Science, Myongji University, San 38-2, Nam-dong, Cheoin-gu, Yongin, Gyeonggi 449-728, Korea. sschoi@mju.ac.kr
Abstract
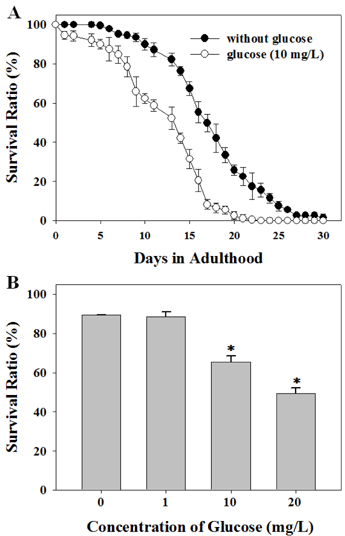
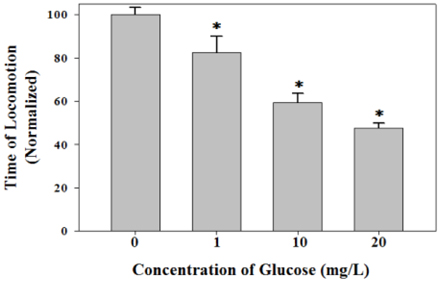
- Diets based on carbohydrates increase rapidly the blood glucose level due to the fast conversion of carbohydrates to glucose. High glucose diets have been known to induce many lifestyle diseases. Here, we demonstrated that high glucose diet shortened the lifespan of Caenorhabditis elegans through apoptosis induction. Control adult groups without glucose diet lived for 30 days, whereas animals fed 10 mg/L of D-glucose lived only for 20 days. The reduction of lifespan by glucose diet showed a dose-dependent profile in the concentration range of glucose from 1 to 20 mg/L. Aging effect of high glucose diet was examined by measurement of response time for locomotion after stimulating movement of the animals by touching. Glucose diet decreased the locomotion capacity of the animals during mid-adulthood. High glucose diets also induced ectopic apoptosis in the body of C. elegans, which is a potent mechanism that can explain the shortened lifespan and aging. Apoptotic cell corpses stained with SYTO 12 were found in the worms fed 10 mg/L of glucose. Mutation of core apoptotic regulatory genes, CED-3 and CED-4, inhibited the reduction of viability induced by high glucose diet, which indicates that these regulators were required for glucose-induced apoptosis or lifespan shortening. Thus, we conclude that high glucose diets have potential for inducing ectopic apoptosis in the body, resulting in a shortened lifespan accompanied with loss of locomotion capacity.
Keyword
MeSH Terms
Figure
Reference
-
1. Lin MH, Wu MC, Lu S, Lin J. Glycemic index, glycemic load and insulinemic index of Chinese starchy foods. World J Gastroenterol. 2010. 16:4973–4979.
Article2. Brand-Miller JC, Holt SH, Pawlak DB, McMillan J. Glycemic index and obesity. Am J Clin Nutr. 2002. 76:281S–285S.
Article3. Natarajan P, Ray KK, Cannon CP. High-density lipoprotein and coronary heart disease: current and future therapies. J Am Coll Cardiol. 2010. 55:1283–1299.4. Kendall CW, Josse AR, Esfahani A, Jenkins DJ. Nuts, metabolic syndrome and diabetes. Br J Nutr. 2010. 104:465–473.
Article5. Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001. 131:3109S–3120S.
Article6. Minich DM, Bland JS. Dietary management of the metabolic syndrome beyond macronutrients. Nutr Rev. 2008. 66:429–444.
Article7. Choi SS, Rhee WJ, Park TH. Inhibition of human cell apoptosis by silkworm hemolymph. Biotechnol Prog. 2002. 18:874–878.
Article8. Choi SS, Rhee WJ, Park TH. Beneficial effect of silkworm hemolymph on a CHO cell system: Inhibition of apoptosis and increase of EPO production. Biotechnol Bioeng. 2005. 91:793–800.
Article9. Choi SS, Rhee WJ, Kim EJ, Park TH. Enhancement of recombinant protein production in Chinese hamster ovary cells through anti-apoptosis engineering using 30Kc6 gene. Biotechnol Bioeng. 2006. 95:459–467.
Article10. Ellis HM, Horvitz HR. Genetic control of programmed cell death in the nematode C. elegans. Cell. 1986. 44:817–829.
Article11. Yuan JY, Horvitz HR. The Caenorhabditis elegans genes ced-3 and ced-4 act cell autonomously to cause programmed cell death. Dev Biol. 1990. 138:33–41.
Article12. Hengartner MO, Ellis RE, Horvitz HR. Caenorhabditis elegans gene ced-9 protects cells from programmed cell death. Nature. 1992. 356:494–499.
Article13. Hengartner MO, Horvitz HR. Activation of C. elegans cell death protein CED-9 by an amino-acid substitution in a domain conserved in Bcl-2. Nature. 1994. 369:318–320.
Article14. Potts MB, Cameron S. Cell lineage and cell death: Caenorhabditis elegans and cancer research. Nat Rev Cancer. 2011. 11:50–58.
Article15. Lamitina T. Functional genomic approaches in C. elegans. Methods Mol Biol. 2006. 351:127–138.16. Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003. 300:1142–1145.
Article17. Gumienny TL, Lambie E, Hartwieg E, Horvitz HR, Hengartner MO. Genetic control of programmed cell death in the Caenorhabditis elegans hermaphrodite germline. Development. 1999. 126:1011–1022.
Article18. Lee SJ, Murphy CT, Kenyon C. Glucose shortens the life span of C. elegans by downregulating DAF-16/FOXO activity and aquaporin gene expression. Cell Metab. 2009. 10:379–391.
Article19. Zhao H, Liu G, Wang Q, Ding L, Cai H, Jiang H, Xin Z. Effect of ghrelin on human endothelial cells apoptosis induced by high glucose. Biochem Biophys Res Commun. 2007. 362:677–681.
Article20. Partridge L. Some highlights of research on aging with invertebrates, 2010. Aging Cell. 2011. 10:5–9.
Article21. Hoffman DJ. Early nutrition and adult health: perspectives for international and community nutrition programs and policies. Nutr Res Pract. 2010. 4:449–454.
Article22. Cheong MC, Na K, Kim H, Jeong SK, Joo HJ, Chitwood DJ, Paik YK. A potential biochemical mechanism underlying the influence of sterol deprivation stress on Caenorhabditis elegans longevity. J Biol Chem. 2011. 286:7248–7256.
Article23. Steinkraus KA, Smith ED, Davis C, Carr D, Pendergrass WR, Sutphin GL, Kennedy BK, Kaeberlein M. Dietary restriction suppresses proteotoxicity and enhances longevity by an hsf-1-dependent mechanism in Caenorhabditis elegans. Aging Cell. 2008. 7:394–404.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Moringa oleifera Prolongs Lifespan via DAF-16/FOXO Transcriptional Factor in Caenorhabditis elegans
- The Longevity Properties of 1,2,3,4,6-Penta-O-Galloyl-beta-D-Glucose from Curcuma longa in Caenorhabditis elegans
- Mechanisms of aging-related proteinopathies in Caenorhabditis elegans
- Genistein from Vigna angularis Extends Lifespan in Caenorhabditis elegans
- Acanthopanax sessiliflorus stem confers increased resistance to environmental stresses and lifespan extension in Caenorhabditis elegans