Nutr Res Pract.
2010 Dec;4(6):492-498.
Low HDL cholesterol is associated with increased atherogenic lipoproteins and insulin resistance in women classified with metabolic syndrome
- Affiliations
-
- 1Department of Nutritional Sciences, University of Connecticut, 3624 Horsebarn Road Ext, Storrs, CT 06269, USA. maria-luz.fernandez@uconn.edu
- 2Department of Emergency Medicine, University of Florida, Jacksonville FL, USA.
- 3Department of Family Medicine, University of California, Irvine, CA, USA.
- 4MetaProteomics LLC, Gig Harbor, WA, USA.
Abstract
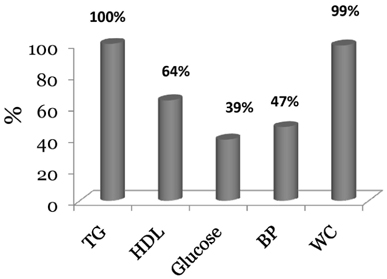
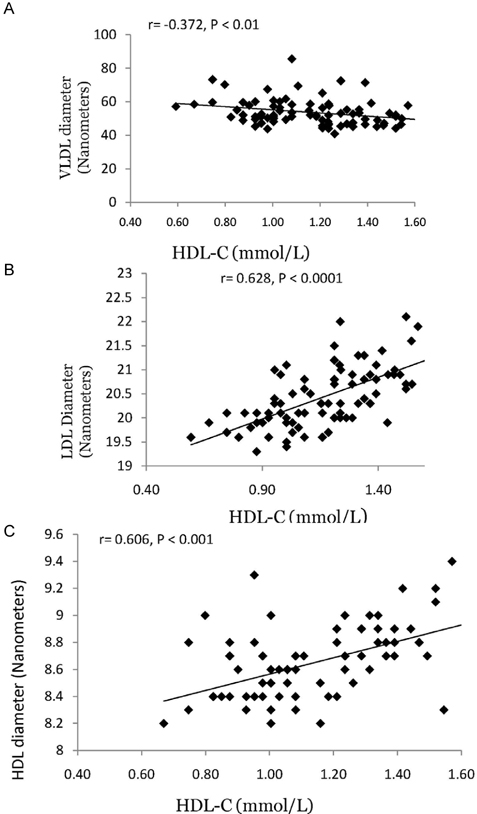
- Both metabolic syndrome (MetS) and elevated LDL cholesterol (LDL-C) increase the risk for cardiovascular disease (CVD). We hypothesized that low HDL cholesterol (HDL-C) would further increase CVD risk in women having both conditions. To assess this, we recruited 89 women with MetS (25-72 y) and LDL-C > or = 2.6 mmol/L. To determine whether plasma HDL-C concentrations were associated with dietary components, circulating atherogenic particles, and other risk factors for CVD, we divided the subjects into two groups: high HDL-C (H-HDL) (> or = 1.3 mmol/L, n = 32) and low HDL-C (L-HDL) (< 1.3 mmol/L, n = 57). Plasma lipids, insulin, adiponectin, apolipoproteins, oxidized LDL, Lipoprotein(a), and lipoprotein size and subfractions were measured, and 3-d dietary records were used to assess macronutrient intake. Women with L-HDL had higher sugar intake and glycemic load (P < 0.05), higher plasma insulin (P < 0.01), lower adiponectin (P < 0.05), and higher numbers of atherogenic lipoproteins such as large VLDL (P < 0.01) and small LDL (P < 0.001) than the H-HDL group. Women with L-HDL also had larger VLDL and both smaller LDL and HDL particle diameters (P < 0.001). HDL-C was positively correlated with LDL size (r = 0.691, P < 0.0001) and HDL size (r = 0.606, P < 0.001), and inversely correlated with VLDL size (r = -0.327, P < 0.01). We concluded that L-HDL could be used as a marker for increased numbers of circulating atherogenic lipoproteins as well as increased insulin resistance in women who are already at risk for CVD.
Keyword
MeSH Terms
Figure
Reference
-
1. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002. 106:3143–3421.2. Fernandez ML. The Metabolic Syndrome. Nutr Rev. 2007. 65:S30–S34.
Article3. Zhang B, Menzin J, Friedman M, Korn JR, Burge RT. Predicted coronary risk for adults with coronary heart disease and low HDL-C: an analysis from the US National Health and Nutrition Examination Survey. Curr Med Res Opin. 2008. 24:2711–2717.
Article4. Lee JY, Parks JS. ATP-binding cassette transporter AI and its role in HDL formation. Curr Opin Lipidol. 2005. 16:19–25.
Article5. Rosenblat M, Aviram M. Paraoxonases role in the prevention of cardiovascular diseases. Biofactors. 2009. 35:98–104.
Article6. Fitzgerald ML, Mujawar Z, Tamehiro N. ABC transporters, atherosclerosis and inflammation. Atherosclerosis. 2010. 211:361–370.
Article7. Murphy AJ, Chin-Dusting JP, Sviridov D, Woollard KJ. The anti inflammatory effects of high density lipoproteins. Curr Med Chem. 2009. 16:667–675.
Article8. Navab M, Anantharamaiah GM, Reddy ST, Van Lenten BJ, Fogelman AM. HDL as a biomarker, potential therapeutic target, and therapy. Diabetes. 2009. 58:2711–2717.
Article9. Saddar S, Mineo C, Shaul PW. Signaling by the high-affinity HDL receptor scavenger receptor B type I. Arterioscler Thromb Vasc Biol. 2010. 30:144–150.
Article10. Volek JS, Fernandez ML, Feinman RD, Phinney SD. Dietary carbohydrate restriction induces a unique metabolic state positively affecting atherogenic dyslipidemia, fatty acid partitioning and metabolic syndrome. Prog Lipid Res. 2008. 47:307–318.
Article11. Al-Sarraj T, Saadi H, Calle MC, Volek JS, Fernandez ML. Carbohydrate restriction as a first-line dietary intervention therapy effectively reduces the biomarkers of metabolic syndrome in Emirati adults. J Nutr. 2009. 139:1667–1676.
Article12. Mutungi G, Ratliff J, Puglisi M, Torres-Gonzalez M, Vaishnav U, Leite JO, Quann E, Volek JS, Fernandez ML. Dietary cholesterol from eggs increases HDL cholesterol in overweight men consuming a carbohydrate restricted diet. J Nutr. 2008. 138:272–276.
Article13. Wood RJ, Volek JS, Liu Y, Shachter NS, Contois JH, Fernandez ML. Carbohydrate restriction alters lipoprotein metabolism by modifying VLDL, LDL and HDL subfraction distribution and size in overweight men. J Nutr. 2006. 136:384–389.
Article14. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972. 18:499–502.
Article15. Haffner SM, Miettinen H, Stern MP. The homeostasis model in the San Antonio Heart Study. Diabetes Care. 1997. 20:1087–1092.
Article16. Liu MY, Xydakis AM, Hoogeveen RC, Jones PH, Smith EO, Nelson KW, Ballantyne CM. Multiplexed analysis of biomarkers related to obesity and the metabolic syndrome in human plasma, using the Luminex-100 system. Clin Chem. 2005. 51:1102–1109.
Article17. Hulthe J, Fagerberg B. Circulating oxidized LDL is associated with subclinical atherosclerosis development and inflammatory cytokines (AIR Study). Arterioscler Thromb Vasc Biol. 2002. 22:1162–1167.
Article18. Wood RJ, Volek JS, Davis SR, Dell'Ova C, Fernandez ML. Effects of a carbohydrate-restricted diet on emerging plasma markers for cardiovascular disease. Nutr Metab (Lond). 2006. 3:19.
Article19. Ratliff JC, Mutungi G, Puglisi M, Volek JS, Fernandez ML. Eggs modulate the inflammatory response to carbohydrate restricted diets in overweight men. Nutr Metab (Lond). 2008. 5:6.
Article20. Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, Bowling AC, Newman HC, Jenkins AL, Goff DV. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981. 34:362–366.
Article21. Lakshman R, Garige M, Gong M, Leckey L, Varatharajalu R, Zakhari S. Is alcohol beneficial or harmful for cardioprotection? Genes Nutr. 2010. 5:111–120.
Article22. Denke MA. Dietary fat, fatty acids and their effects on lipoproteins. Curr Atheroscler Rep. 2006. 8:466–471.
Article23. Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, Retzlaff BM, Knopp RH, Brunzell JD, Kahn SE. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003. 46:459–469.
Article24. Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000. 20:1595–1599.
Article25. Marotta T, Russo BE, Ferrara LA. Triglyceride-to-HDL cholesterol ratio and metabolic syndrome as contributors to cardiovascular risk in overweight patients. Obesity (Silver Spring). 2010. 18:1608–1613.
Article26. Shachter NS. Apolipoproteins C-I and C-III as important modulators of lipoprotein metabolism. Curr Opin Lipidol. 2001. 12:297–304.
Article27. Austin MA, King MC, Vranizan KM, Krauss RM. Atherogenic lipoprotein phenotype. A proposed genetic marker for coronary heart disease risk. Circulation. 1990. 82:495–506.
Article28. Yamashita T, Sasahara T, Pomeroy SE, Collier G, Nestel PJ. Arterial compliance, blood pressure, plasma leptin, and plasma lipids in women are improved with weight reduction equally with a meat-based diet and a plant-based diet. Metabolism. 1998. 47:1308–1314.
Article29. Millar JS, Packard CJ. Heterogeneity of apolipoprotein B-100-containing lipoproteins: what we have learnt from kinetic studies. Curr Opin Lipidol. 1998. 9:197–202.
Article30. Gianturco SH, Ramprasad MP, Song R, Li R, Brown ML, Bradley WA. Apolipoprotein B-48 or Its Apolipoprotein B-100 Equivalent Mediates the Binding of Triglyceride-Rich lipoproteins to Their Unique Human Monocyte-Macrophage Receptor. Arterioscler Thromb Vasc Biol. 1998. 18:968–976.
Article31. Zambon A, Bertocco S, Vitturi N, Polentarutti V, Vianello D, Crepaldi G. Relevance of hepatic lipase to the metabolism of triacylglycerol-rich lipoproteins. Biochem Soc Trans. 2003. 31:1070–1074.
Article32. Rashid S, Watanabe T, Sakaue T, Lewis GF. Mechanisms of HDL lowering in insulin resistant, hypertriglyceridemic states: the combined effect of HDL triglyceride enrichment and elevated hepatic lipase activity. Clin Biochem. 2003. 36:421–429.
Article33. Gardner CD, Fortmann SP, Krauss RM. Association of small low-density lipoprotein particles with the incidence of coronary artery disease in men and women. JAMA. 1996. 276:875–881.
Article34. Krauss RM. Relationship of intermediate and low-density lipoprotein subspecies to risk of coronary heart disease. Am Heart J. 1987. 113:578–582.
Article35. Tall AR. Cholesterol efflux pathways and other potential mechanisms involved in the athero-protective effect of high density lipoproteins. J Intern Med. 2008. 263:256–273.
Article36. Seman LJ, McNamara JR, Schaefer EJ. Lipoprotein(a), homocysteine, and remnantlike particles: emerging risk factors. Curr Opin Cardiol. 1999. 14:186–191.
Article37. Itabe H, Mori M, Fujimoto Y, Higashi Y, Takano T. Minimally modified LDL is an oxidized LDL enriched with oxidized phosphatidyl cholines. J Biochem. 2003. 134:459–465.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Causes and Consequences of Low Levels of High Density Lipoproteins in Patients with Diabetes
- Metabolic Syndrome in Children
- Correlation of the Serum Testosterone Level with Insulin Resistance and Metabolic Syndrome in Patients of Erectile Dysfunction and Benign Prostatic Hyperplasia
- Effect of zinc supplementation on insulin resistance and metabolic risk factors in obese Korean women
- Serum Ferritin and Metabolic Syndrome in Perimenopausal and Postmenopausal Women