Korean J Urol.
2008 Jul;49(7):585-591.
Significance of Neuroendocrine Cell Differentiation in Specimens from Patients with Prostate Cancer
- Affiliations
-
- 1Departments of Urology, Gangneung Asan Hospital, College of Medicine, Ulsan University, Korea. jypark@gnah.co.kr
- 2Departments of Pathology, Gangneung Asan Hospital, College of Medicine, Ulsan University, Korea.
- 3Department of Preventive Medicine, College of Medicine, Kwandong University, Gangneung, Korea.
Abstract
-
PURPOSE: The neuroendocrine cell(NEC) is one of the constitutional cells found in the prostate gland; these cells secret neurotransmitters. These neuroendocrine products have been associated with prostate cancer progression. We evaluated the significance of neuroendocrine differentiation(NED) in radical prostatectomy specimens.
MATERIALS AND METHODS
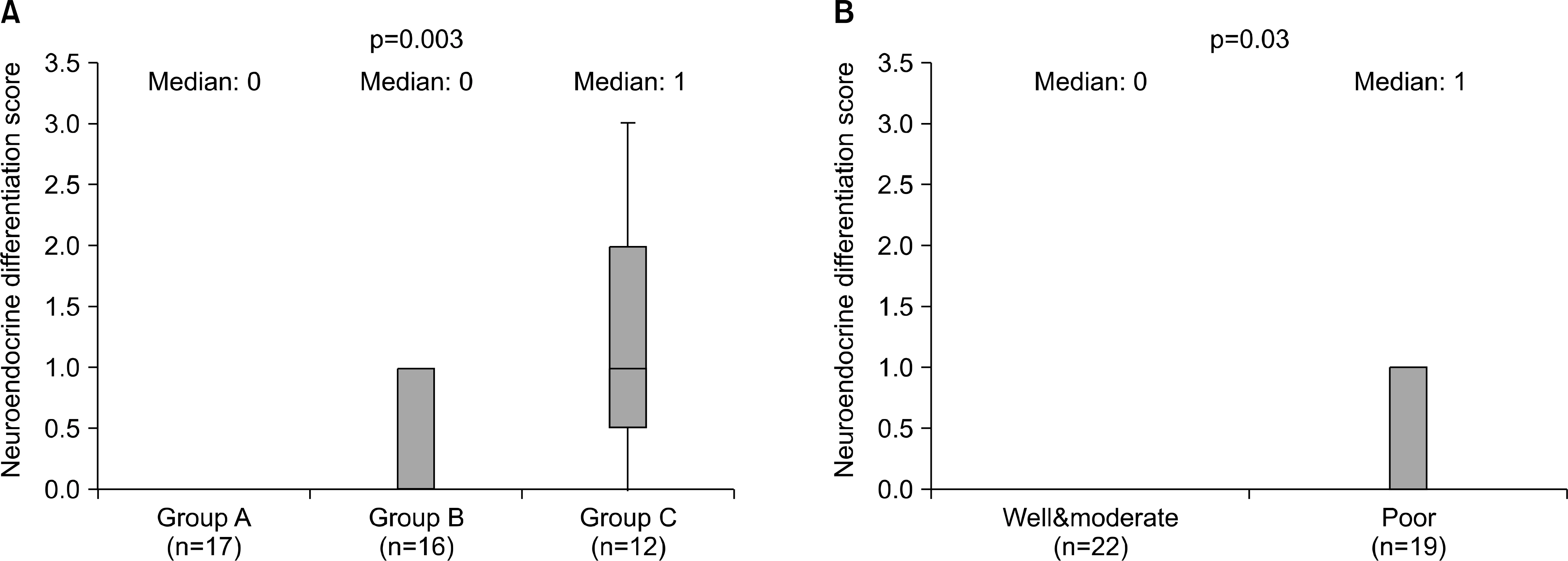
We studied 45 patients who underwent bilateral pelvic lymphadenectomy and radical prostatectomy. The patients were classified into three groups according to their pathological stage. Group A included cases with organ confined tumors, Group B local advanced tumors and Group C cases had any T stage and lymph node metastasis. The cellular expression of chromogranin A in matched samples from the same patients was evaluated by immunohistochemical staining using commercially available monoclonal antibodies.
RESULTS
Sixteen(35.6%) tumors had chromogranin A stained cells. Chromogranin A immunoreactivity was greatest in cases with lymph node involvement(75.0%) compared to those with primary prostate cancer(5.9% in group A and 37.5% in group B). Pathologically advanced tumors or tumors with the highest histological grades were associated with increased NED. The median staining score was 0 in Group A, 0 in Group B and 1 in Group C. The logistic regression analysis the odds ratio for group C cases showed a relative risk of 32.07(95% CI: 2.783-369.416) for NED compared to Group A. An increased prostate-specific antigen(PSA) and Gleason score were also associated with the NED.
CONCLUSIONS
The degree of NEC immunohistochemical staining using the chromogranin A monoclonal antibody was marginally useful for predicting the outcome in prostate cancer patients after radical prostatectomy, especially in node positive patients. However, it is important to determine a therapeutic plan for patients with low PSA and internal organ metastasis.
MeSH Terms
Figure
Reference
-
1.Bonkhoff H., Stein U., Remberger K. Multidirectional differentiation in the normal, hyperplastic, and neoplastic human prostate: simultaneous demonstration of cell-specific epithelial markers. Hum Pathol. 1994. 25:42–6.
Article2.Theodorescu D., Broder SR., Boyd JC., Mills SE., Frierson HF Jr. Cathepsin D and chromogranin A as predictors of long term disease specific survival after radical prostatectomy for localized carcinoma of the prostate. Cancer. 1997. 80:2109–19.
Article3.Cohen RJ., Glezerson G., Haffejee Z. Neuro-endocrine cells-a new prognostic parameter in prostate cancer. Br J Urol. 1991. 68:258–62.
Article4.Krijnen JL., Janssen PJ., Ruizeveld de Winter JA., van Krimpen H., Schroder FH., van der Kwast TH. Do neuroendocrine cells in human prostate cancer express androgen receptor? Histochemistry. 1993. 100:393–8.
Article5.Ahlgren G., Pedersen K., Lundberg S., Aus G., Hugosson J., Abrahamsson PA. Regressive changes and neuroendocrine differentiation in prostate cancer after neoadjuvant hormonal treatment. Prostate. 2000. 42:274–9.
Article6.Bostwick DG., Qian J., Pacelli A., Zincke H., Blute M., Ber-gstralh EJ, et al. Neuroendocrine expression in node positive prostate cancer: correlation with systemic progression and patient survival. J Urol. 2002. 168:1204–11.
Article7.Bostwick DG., Grignon DJ., Hammond ME., Amin MB., Cohen M., Crawford D, et al. Prognostic factors in prostate cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000. 124:995–1000.8.Shariff AH., Ather MH. Neuroendocrine differentiation in prostate cancer. Urology. 2006. 68:2–8.
Article9.Abrahamsson PA., Wadstrom LB., Alumets J., Falkmer S., Grimelius L. Peptide-hormone and serotonin-immunoreactive tumour cells in carcinoma of the prostate. Pathol Res Pract. 1987. 182:298–307.
Article10.Weinstein MH., Partin AW., Veltri RW., Epstein JI. Neuroendocrine differentiation in prostate cancer: enhanced prediction of progression after radical prostatectomy. Hum Pathol. 1996. 27:683–7.
Article11.Kim YJ., Choe G., Hong SK., Byun SS., Lee SE., Lee NK. Pathological characteristics of neuroendocrine cell differentiation in prostate cancer. Korean J Urol. 2007. 48:143–51.
Article12.Speights VO Jr., Cohen MK., Riggs MW., Coffield KS., Keegan G., Arber DA. Neuroendocrine stains and proliferative indices of prostatic adenocarcinomas in transurethral resection samples. Br J Urol. 1997. 80:281–6.13.Aprikian AG., Cordon-Cardo C., Fair WR., Reuter VE. Characterization of neuroendocrine differentiation in human benign prostate and prostatic adenocarcinoma. Cancer. 1993. 71:3952–65.
Article14.Allen FJ., Van Velden DJ., Heyns CF. Are neuroendocrine cells of practical value as an independent prognostic parameter in prostate cancer? Br J Urol. 1995. 75:751–4.
Article15.Pruneri G., Galli S., Rassi RS., Roncalli M., Coggi G., Ferrari A, et al. Chromogranin A and B secrerogranin II in prostatic adenocarcinomas: neuroendocrine expression in patients untreated and treated with androgen deprivation therapy. Prostate. 1998. 34:113–20.16.Talpin ME., George DJ., Halabi S., Sanford B., Febbo PG., Hennessy KT, et al. Prognostic significance of plasma chro-mogeanin A levels in patients with hormone-refractory prostate cancer treated in Cancer and Leukemia Group B 9480 study. Urology. 2005. 66:386–91.17.Bonkhoff H., Wernert N., Dhom G., Remberger K. Relation of endocrine-paracrine cells to cell proliferation in normal, hyperplastic, and neoplastic human prostate. Prostate. 1991. 19:91–8.
Article18.Ambrosini G., Adida C., Altieri DC. A novel antiapoptosis gene, surviving, expressed in cancer lymphoma. Nat Med. 1997. 3:917–21.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Prognostic Significance of Neuroendocrine Differentiation for Treating Prostatic Carcinoma in 699 Cases of Radical Prostatectomy
- Large Cell Neuroendocrine Carcinoma of the Lung: Report of three cases
- Pathological Characteristics of Neuroendocrine Cell Differentiation in Prostate Cancer
- Expression Pattern of Neuroendocrine Cells and Survivin in the Prostate of Rabbits
- The Neuroendocrine Cell in Benign Prostatic Hyperplasia : the Relation of the Neuroendocrine Cell with Total Prostate Volume and Transition Zone Volume