Korean J Urol.
2008 Dec;49(12):1074-1080.
Prognostic Significance of the Tumor Volume and Tumor Percentage for Localized Prostate Cancer
- Affiliations
-
- 1Department of Urology, College of Medicine, Pochon CHA University, Korea.
- 2Department of Urology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea. selee@snubh.org
- 3Department of Pathology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
Abstract
-
PURPOSE: Tumor volume has been thought to be an important predictive factor for significant prostate cancer. We assessed the impact of the tumor volume(TV) and the tumor percentage(TP) of radical prostatectomy specimens on the pathological variables and the oncological outcome.
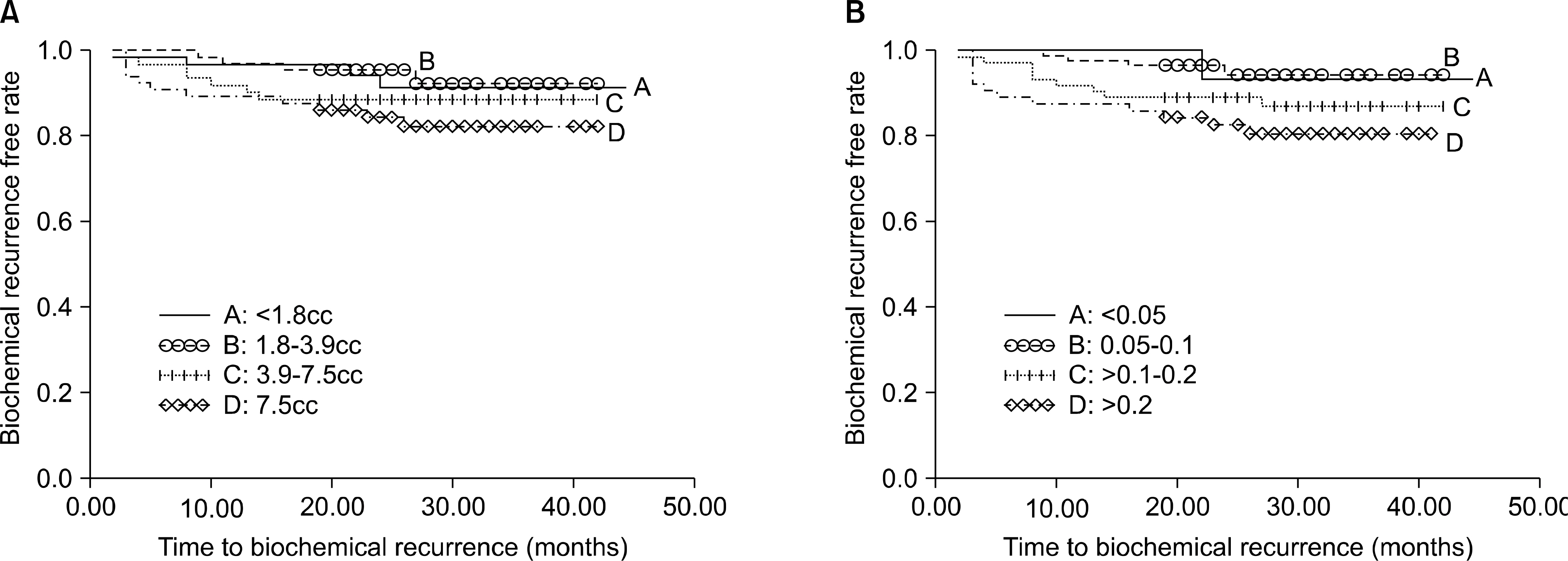
MARERIALS AND METHODS: The tumor percentage and tumor volume were calculated for 525 cases by a single pathologist who determined the volume based on the surface area of the slides involved by tumor of the prostate. Univariate and multivariate logistic regression analyses were used to characterize the association of TP categories(<5%, 5-10%, 11-20% and >20%) and TV(<1.8cc, 1.8-3.7cc, 3.8-7.5cc, >7.5cc) with the clinicopathological variables. Biochemical recurrence(BCR) was estimated using Kaplan-Meier analysis and Cox's hazard regression model.
RESULTS
The mean prostate cancer volume was 6.5+/-8.5cc(median: 3.8, range: 0.04-73.8) and the mean percent tumor composition was 0.17+/-0.19 (median: 0.1, range: 0.01-0.95). A higher tumor volume and a higher tumor percentage were associated with extra-capsular extension(ECE), a positive surgical margin(PSM), a higher pT stage and a higher prostate-specific antigen(PSA) Gleason score(all p<0.05). In addition, TP was the independent predictor of ECE(adjusted odds ratio(OR): 22.66, 95% confidence interval(CI): 1.801-285.079, p=0.016), but the tumor volume was not associated with ECE on the multivariate logistic analyses. On the Kaplan-Meier analysis, but not on the Cox-hazard analyses, the TP did demonstrate a significant association with biochemical recurrence(p=0.035), yet the TV did not reach statistical significance(p=0.190).
CONCLUSIONS
Our data indicates that the tumor percentage had a significant effect on the BCR on the Kaplan-Meier analysis. The tumor percentage rather than the tumor volume might be more useful to predict the prognosis of prostate cancer.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Chun FK, Graefen M, Zacharias M, Haese A, Steuber T, Schlomm T, et al. Anatomic radical retropubic prostatectomy-longterm recurrence-free survival rates for localized prostate cancer. World J Urol. 2006; 24:273–80.
Article2. Kupelian PA, Katcher J, Levin HS, Klein EA. Stage T1–2 prostate cancer: a multivariate analysis of factors affecting biochemical and clinical failures after radical prostatectomy. Int J Radiat Oncol Biol Phys. 1997; 37:1043–52.3. Walsh PC, Partin AW, Epstein JI. Cancer control and quality of life following anatomical radical retropubic prostatectomy: results at 10 years. J Urol. 1994; 152:1831–6.
Article4. Zincke H, Bergstralh EJ, Blute ML, Myers RP, Barrett DM, Lieber MM, et al. Radical prostatectomy for clinically localized prostate cancer: longterm results of 1,143 patients from a single institution. J Clin Oncol. 1994; 12:2254–63.
Article5. Bastian PJ, Gonzalgo ML, Aronson WJ, Terris MK, Kane CJ, Amling CL, et al. Clinical and pathologic outcome after radical prostatectomy for prostate cancer patients with a preoperative Gleason sum of 8 to 10. Cancer. 2006; 107:1265–72.
Article6. Carver BS, Bianco FJ Jr, Scardino PT, Eastham JA. Longterm outcome following radical prostatectomy in men with clinical stage T3 prostate cancer. J Urol. 2006; 176:564–8.
Article7. Ross PL, Gerigk C, Gonen M, Yossepowitch O, Cagiannos I, Sogani PC, et al. Comparisons of nomograms and urologists' predictions in prostate cancer. Semin Urol Oncol. 2002; 20:82–8.
Article8. Freedland SJ, Mangold LA, Walsh PC, Partin AW. The prostatic specific antigen era is alive and well: prostatic specific antigen and biochemical progression following radical prostatectomy. J Urol. 2005; 174:1276–81.
Article9. McNeal JE, Bostwick DG, Kindrachuk RA, Redwine EA, Freiha FS, Stamey TA. Patterns of progression in prostate cancer. Lancet. 1986; 1:60–3.
Article10. McNeal JE, Villers AA, Redwine EA, Freiha FS, Stamey TA. Capsular penetration in prostate cancer. Significance for natural history and treatment. Am J Surg Pathol. 1990; 14:240–7.
Article11. Guzzo TJ, Vira MA, Neway W, Hwang WT, Tomaszewski J, VanArsdalen K, et al. Minimal tumor volume may provide additional prognostic information in good performance patients after radical prostatectomy. Urology. 2007; 69:1147–51.
Article12. Merrill MM, Lane BR, Reuther AM, Zhou M, Magi-Galluzzi C, Klein EA. Tumor volume does not predict for biochemical recurrence after radical prostatectomy in patients with surgical Gleason score 6 or less prostate cancer. Urology. 2007; 70:294–8.
Article13. Stamey TA, McNeal JE, Yemoto CM, Sigal BM, Johnstone IM. Biological determinants of cancer progression in men with prostate cancer. JAMA. 1999; 281:1395–400.
Article14. Epstein JI, Carmichael M, Partin AW, Walsh PC. Is tumor volume an independent predictor of progression following radical prostatectomy? A multivariate analysis of 185 clinical stage B adenocarcinomas of the prostate with 5 years of follow up. J Urol. 1993; 149:1478–81.15. Kikuchi E, Scardino PT, Wheeler TM, Slawin KM, Ohori M. Is tumor volume an independent prognostic factor in clinically localized prostate cancer? J Urol. 2004; 172:508–11.
Article16. Salomon L, Levrel O, Anastasiadis AG, Irani J, De La Taille A, Saint F, et al. Prognostic significance of tumor volume after radical prostatectomy: a multivariate analysis of pathological prognostic factors. Eur Urol. 2003; 43:39–44.
Article17. Ohori M, Wheeler TM, Kattan MW, Goto Y, Scardino PT. Prognostic significance of positive surgical margins in radical prostatectomy specimens. J Urol. 1995; 154:1818–24.
Article18. D'Amico AV, Cote K, Loffredo M, Renshaw AA, Chen MH. Pretreatment predictors of time to cancer specific death after prostate specific antigen failure. J Urol. 2003; 169:1320–4.19. McNeal JE. Origin and development of carcinoma in the prostate. Cancer. 1969; 23:24–34.
Article20. Lee KR, Cheon J. Clinical significance of calculated prostate cancer volume as the predictor of pathologic stage. Korean J Urol. 2001; 42:821–7.21. Freedland SJ, Isaacs WB, Platz EA, Terris MK, Aronson WJ, Amling CL, et al. Prostate size and risk of high-grade, advanced prostate cancer and biochemical progression after radical prostatectomy: a search database study. J Clin Oncol. 2005; 23:7546–54.
Article22. Rampersaud EN, Sun L, Moul JW, Madden J, Freedland SJ. Percent tumor involvement and risk of biochemical progression after radical prostatectomy. J Urol. 2008; 180:571–6.
Article23. Noguchi M, Stamey TA, McNeal JE, Yemoto CE. Assessment of morphometric measurements of prostate carcinoma volume. Cancer. 2000; 89:1056–64.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Significance of Calculated Prostate Cancer Volume as the Predictor of Pathologic Stage
- Prostate Volume has Prognostic Value Only in Pathologic T2 Radical Prostatectomy Specimens
- Efficacy of Radical Retropubic Prostatectomy in Patients with Clinically Localized Prostate Cancer and a Biopsy Gleason Score of 8 or Higher
- Efficacy of Radical Retropubic Prostatectomy as the Primary Treatment for Patients with Clinically Localized Prostate Cancer and a Serum PSA Level >or=20ng/ml
- The Effect of Tumor-Prostate Ratio on Biochemical Recurrence after Radical Prostatectomy