World J Mens Health.
2016 Aug;34(2):123-128. 10.5534/wjmh.2016.34.2.123.
The Effect of Tumor-Prostate Ratio on Biochemical Recurrence after Radical Prostatectomy
- Affiliations
-
- 1Department of Urology, Inje University College of Medicine, Busan, Korea.
- 2Department of Urology, Yonsei University College of Medicine, Seoul, Korea. YOUNGD74@yuhs.ac
- KMID: 2349848
- DOI: http://doi.org/10.5534/wjmh.2016.34.2.123
Abstract
- PURPOSE
Prostate tumor volume calculated after surgery using pathologic tissue has been shown to be an independent risk factor for biochemical recurrence. Nonetheless, prostate size varies among individuals, regardless of the presence or absence of cancer. We assumed to be lower margin positive rate in the surgical operation, when the prostate volume is larger and the tumor lesion is same. Thus, we defined the tumor-prostate ratio in the ratio of tumor volume to prostate volume. In order to compensate the prostate tumor volume, the effect of tumor-prostate ratio on biochemical recurrence was examined.
MATERIALS AND METHODS
This study included 251 patients who underwent open retropubic radical prostatectomy for prostate cancer in a single hospital. We analyzed the effects of tumor volume and tumor-prostate ratio, as well as the effects of known risk factors for biochemical recurrence, on the duration of disease-free survival.
RESULTS
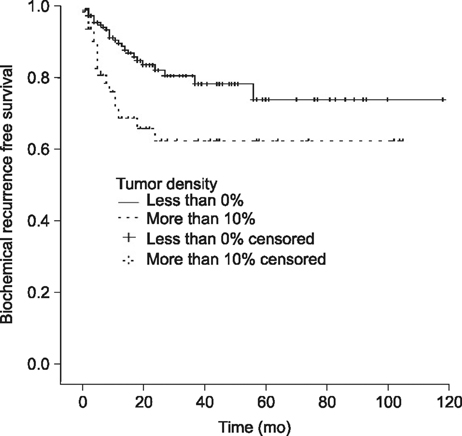
In the univariate analysis, the risk factors that significantly impacted disease-free survival time were found to be a prostate-specific antigen level ≥10 ng/mL, a tumor volume ≥5 mL, tumor-prostate ratio ≥10%, tumor capsular invasion, lymph node invasion, positive surgical margins, and seminal vesicle invasion. In the multivariate analysis performed to evaluate the risk factors found to be significant in the univariate analysis, positive surgical margins (hazard ratio=3.066) and a tumor density ≥10% (hazard ratio=1.991) were shown to be significant risk factors for biochemical recurrence.
CONCLUSIONS
Tumor-prostate ratio, rather than tumor volume, should be regarded as a significant risk factor for biochemical recurrence.
Keyword
MeSH Terms
Figure
Reference
-
1. Stamey TA, McNeal JE, Yemoto CM, Sigal BM, Johnstone IM. Biological determinants of cancer progression in men with prostate cancer. JAMA. 1999; 281:1395–1400.
Article2. Nicholson B, Theodorescu D. Angiogenesis and prostate cancer tumor growth. J Cell Biochem. 2004; 91:125–150.
Article3. Epstein JI, Carmichael M, Partin AW, Walsh PC. Is tumor volume an independent predictor of progression following radical prostatectomy? A multivariate analysis of 185 clinical stage B adenocarcinomas of the prostate with 5 years of followup. J Urol. 1993; 149:1478–1481.
Article4. Bostwick DG, Grignon DJ, Hammond ME, Amin MB, Cohen M, Crawford D, et al. Prognostic factors in prostate cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000; 124:995–1000.5. Korman HJ, Leu PB, Goldstein NS. A prospective comparison of anatomic radical perineal and retropubic prostatectomy specimens: are surgical margins equivalent? Prostate Cancer Prostatic Dis. 2000; 3:S22.
Article6. Brown JA, Garlitz C, Gomella LG, Hubosky SG, Diamond SM, McGinnis D, et al. Pathologic comparison of laparoscopic versus open radical retropubic prostatectomy specimens. Urology. 2003; 62:481–486.
Article7. Rassweiler J, Seemann O, Schulze M, Teber D, Hatzinger M, Frede T. Laparoscopic versus open radical prostatectomy: a comparative study at a single institution. J Urol. 2003; 169:1689–1693.
Article8. McNeal JE. Cancer volume and site of origin of adenocarcinoma in the prostate: relationship to local and distant spread. Hum Pathol. 1992; 23:258–266.
Article9. Nelson BA, Shappell SB, Chang SS, Wells N, Farnham SB, Smith JA Jr, et al. Tumour volume is an independent predictor of prostate-specific antigen recurrence in patients undergoing radical prostatectomy for clinically localized prostate cancer. BJU Int. 2006; 97:1169–1172.
Article10. Noguchi M, Stamey TA, McNeal JE, Yemoto CM. Preoperative serum prostate specific antigen does not reflect biochemical failure rates after radical prostatectomy in men with large volume cancers. J Urol. 2000; 164:1596–1600.
Article11. Marks RA, Koch MO, Lopez-Beltran A, Montironi R, Juliar BE, Cheng L. The relationship between the extent of surgical margin positivity and prostate specific antigen recurrence in radical prostatectomy specimens. Hum Pathol. 2007; 38:1207–1211.
Article12. Alschibaja M, Wegner M, Massmann J, Funk A, Hartung R, Paul R. Prostate cancer volume: can it be predicted preoperatively? Urol Int. 2005; 75:354–359.13. Eichelberger LE, Koch MO, Eble JN, Ulbright TM, Juliar BE, Cheng L. Maximum tumor diameter is an independent predictor of prostate-specific antigen recurrence in prostate cancer. Mod Pathol. 2005; 18:886–890.
Article14. Chun FK, Briganti A, Jeldres C, Gallina A, Erbersdobler A, Schlomm T, et al. Tumour volume and high grade tumour volume are the best predictors of pathologic stage and biochemical recurrence after radical prostatectomy. Eur J Cancer. 2007; 43:536–543.
Article15. Humphrey PA, Vollmer RT. Percentage carcinoma as a measure of prostatic tumor size in radical prostatectomy tissues. Mod Pathol. 1997; 10:326–333.16. Srigley JR. Key issues in handling and reporting radical prostatectomy specimens. Arch Pathol Lab Med. 2006; 130:303–317.
Article17. Renshaw AA, Chang H, D'Amico AV. Estimation of tumor volume in radical prostatectomy specimens in routine clinical practice. Am J Clin Pathol. 1997; 107:704–708.
Article18. Noguchi M, Stamey TA, McNeal JE, Yemoto CE. Assessment of morphometric measurements of prostate carcinoma volume. Cancer. 2000; 89:1056–1064.
Article19. Chen ME, Johnston D, Reyes AO, Soto CP, Babaian RJ, Troncoso P. A streamlined three-dimensional volume estimation method accurately classifies prostate tumors by volume. Am J Surg Pathol. 2003; 27:1291–1301.
Article20. Epstein JI, Amin M, Boccon-Gibod L, Egevad L, Humphrey PA, Mikuz G, et al. Prognostic factors and reporting of prostate carcinoma in radical prostatectomy and pelvic lymphadenectomy specimens. Scand J Urol Nephrol Suppl. 2005; (216):34–63.
Article21. Epstein JI, Carmichael M, Walsh PC. Adenocarcinoma of the prostate invading the seminal vesicle: definition and relation of tumor volume, grade and margins of resection to prognosis. J Urol. 1993; 149:1040–1045.
Article22. Ohori M, Wheeler TM, Kattan MW, Goto Y, Scardino PT. Prognostic significance of positive surgical margins in radical prostatectomy specimens. J Urol. 1995; 154:1818–1824.
Article23. Potter SR, Epstein JI, Partin AW. Seminal vesicle invasion by prostate cancer: prognostic significance and therapeutic implications. Rev Urol. 2000; 2:190–195.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Radical Prostatectomy
- Cribriform Pattern at the Surgical Margin is Highly Predictive of Biochemical Recurrence in Patients Undergoing Radical Prostatectomy
- The Impact of Positive Surgical Margins on Biochemical Recurrence after Radical Retropubic Prostatectomy
- Prediction of Biochemical Failure after Radical Prostatectomy for Localized Prostate Cancer
- How can we best manage biochemical failure after radical prostatectomy?