Korean J Urol.
2009 Mar;50(3):211-223.
Diabetes Attenuates Female Genital Sexual Arousal Response via Disruption of Estrogen Action
- Affiliations
-
- 1Department of Biochemistry and Urology, Boston University School of Medicine, Boston, MA 02118, USA. atraish@bu.edu
- 2Departments of Anatomy and Neurobiology, Boston University School of Medicine, Boston, MA 02118, USA.
- 3Department of Urology, Boston University School of Medicine, Boston, MA 02118, USA.
Abstract
-
PURPOSE: Diabetes profoundly and negatively impacts all domains of female sexual function, however, the underlying pathophysiological mechanisms remain unknown. To date, limited studies have been published on the effects of type 1 & type 2 diabetes on female genital sexual arousal and how this may impact overall sexual function. The aim of this review is to discuss the effects of diabetes on female sexual function and insights from laboratory studies on the underlying pathophysiology.
MATERIALS AND METHODS
Using PubMed, we reviewed and evaluated the literature published between 1970 and 2009 and data from our laboratories and others investigating the effects of type 1 and type 2 diabetes on genital sexual arousal responses.
RESULTS
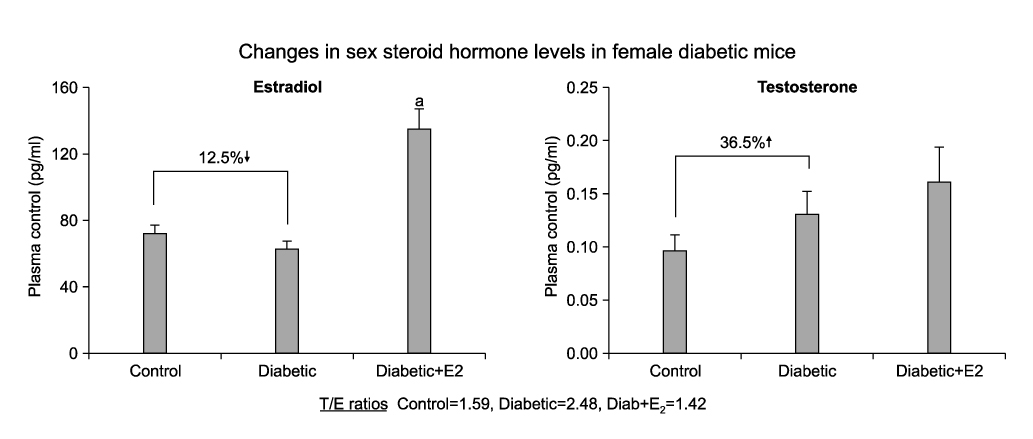
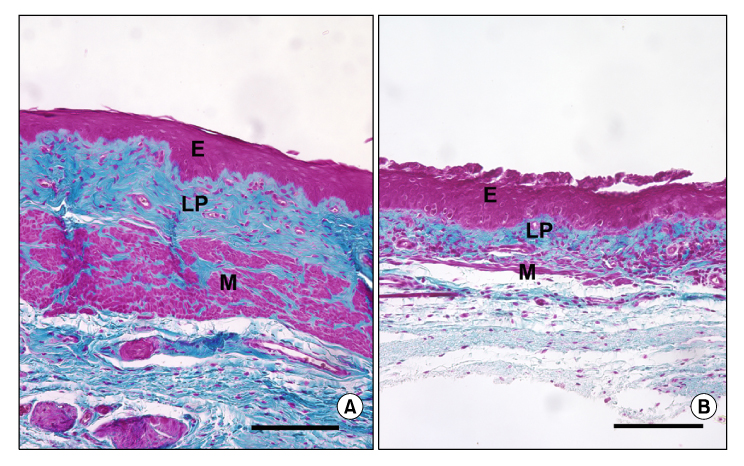
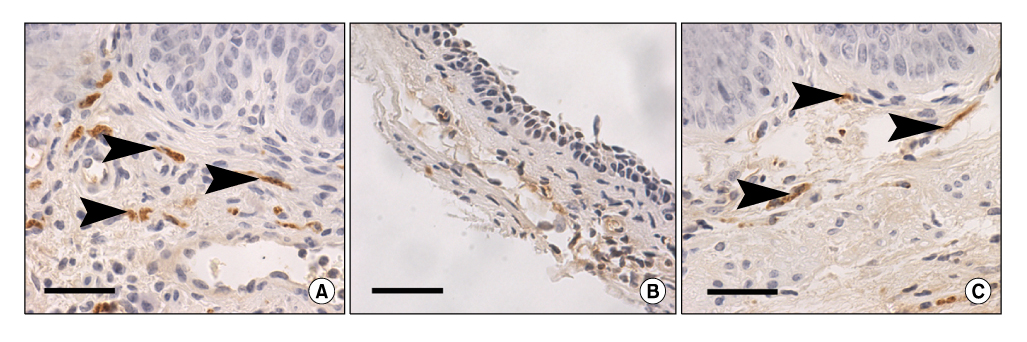
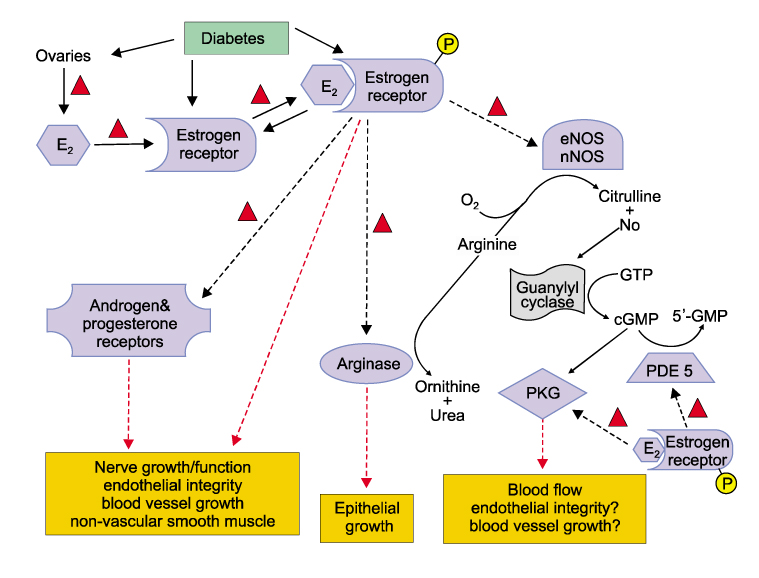
Women with diabetes experience diminished genital arousal, reduced vaginal lubrication, vaginal atrophy, dyspareunia, and increased vaginal infections. Also, a number of studies using type 1 and type 2 diabetic animal models have reported reduced plasma estradiol levels and marked physiological, biochemical and histological changes in genital tissues. In animal studies, diabetes alters genital tissue structure and attenuates expression of the estrogen, progesterone and androgen receptors and alters vaginal and clitoral hemodynamics. Importantly, treatment of diabetic animals with estradiol in the face of persistent hyperglycemia can restore vaginal structure and sex steroid receptor expression.
CONCLUSIONS
Type 1 & type 2 diabetic complications produce significant structural and functional disruptions in genital organs and attenuate genital hemodynamics. In the type 2 animal model, estradiol treatment ameliorates diabetic-induced pathophysiological alterations in genital tissues, such as the vagina. This suggests that estrogen supplementation may be beneficial in restoring diabetes-induced genital pathology.
Keyword
MeSH Terms
-
Animals
Arousal
Atrophy
Clitoris
Diabetes Complications
Diabetes Mellitus
Dyspareunia
Estradiol
Estrogens
Female
Genitalia
Hemodynamics
Humans
Hyperglycemia
Lubrication
Models, Animal
Plasma
Progesterone
Receptors, Androgen
Receptors, Steroid
Testosterone
Vagina
Estradiol
Estrogens
Progesterone
Receptors, Androgen
Receptors, Steroid
Testosterone
Figure
Reference
-
1. Enzlin P, Mathieu C, Vanderschueren D, Demyttenaere K. Diabetes mellitus and female sexuality: a review of 25 years' research. Diabet Med. 1998. 15:809–815.2. Enzlin P, Mathieu C, Van den Bruel A, Bosteels J, Vanderschueren D, Demyttenaere K. Sexual dysfunction in women with type 1 diabetes: a controlled study. Diabetes Care. 2002. 25:672–677.3. Koch PB, Young EW. Diabetes and female sexuality: a review of the literature. Health Care Women Int. 1988. 9:251–262.4. Kolodny RC. Sexual dysfunction in diabetic females. Diabetes. 1971. 20:557–559.5. Fonseca V, Jawa A. Endothelial and erectile dysfunction, diabetes mellitus, and the metabolic syndrome: common pathways and treatments? Am J Cardiol. 2005. 96:13M–18M.6. Bailes BK. Diabetes mellitus and its chronic complications. AORN J. 2002. 76:266–282.7. de Leon EM, Jacober SJ, Sobel JD, Foxman B. Prevalence and risk factors for vaginal Candida colonization in women with type 1 and type 2 diabetes. BMC Infect Dis. 2002. 2:1.8. DeUgarte CM, Berman L, Berman J. Female sexual dysfunction-from diagnosis to treatment. Sex Reprod Menopause. 2004. 2:139–145.9. Grandjean C, Moran B. The impact of diabetes mellitus on female sexual well-being. Nurs Clin North Am. 2007. 42:581–592.10. Rutherford D, Collier A. Sexual dysfunction in women with diabetes mellitus. Gynecol Endocrinol. 2005. 21:189–192.11. Muniyappa R, Norton M, Dunn ME, Banerji MA. Diabetes and female sexual dysfunction: moving beyond "benign neglect". Curr Diab Rep. 2005. 5:230–236.12. Jovanovic L. Finally, it is our turn! Diabetes Care. 2002. 25:787–788.13. Erol B, Tefekli A, Ozbey I, Salman F, Dincag N, Kadioglu A, et al. Sexual dysfunction in type II diabetic females: a comparative study. J Sex Marital Ther. 2002. 28:Suppl 1. 55–62.14. Wincze JP, Albert A, Bansal S. Sexual arousal in diabetic females: physiological and self-report measures. Arch Sex Behav. 1993. 22:587–601.15. Jensen SB. Diabetic sexual dysfunction: a comparative study of 160 insulin treated diabetic men and women and an age-matched control group. Arch Sex Behav. 1981. 10:493–504.16. Schreiner-Engel P, Schiavi RC, Vietorisz D, Smith H. The differential impact of diabetes type on female sexuality. J Psychosom Res. 1987. 31:23–33.17. LeMone P. The physical effects of diabetes on sexuality in women. Diabetes Educ. 1996. 22:361–366.18. Doruk H, Akbay E, Cayan S, Akbay E, Bozlu M, Acar D. Effect of diabetes mellitus on female sexual function and risk factors. Arch Androl. 2005. 51:1–6.19. Abu Ali RM, Al Hajeri RM, Khader YS, Shegem NS, Ajlouni KM. Sexual dysfunction in Jordanian diabetic women. Diabetes Care. 2008. 31:1580–1581.20. Enzlin P, Mathieu C, Van Den Bruel A, Vanderschueren D, Demyttenaere K. Prevalence and predictors of sexual dysfunction in patients with type 1 diabetes. Diabetes Care. 2003. 26:409–414.21. Fatemi SS, Taghavi SM. Evaluation of sexual function in women with type 2 diabetes mellitus. Diab Vasc Dis Res. 2009. 6:38–39.22. Olarinoye J, Olarinoye A. Determinants of sexual function among women with type 2 diabetes in a Nigerian population. J Sex Med. 2008. 5:878–886.23. Mezones-Holguin E, Blümel JE, Huezo M, Vargas R, Castro J, Córdova W, et al. Impact of diabetes mellitus on the sexuality of Peruvian postmenopausal. Gynecol Endocrinol. 2008. 24:470–474.24. Campbell LV, Redelman MJ, Borkman M, McLay JG, Chisholm DJ. Factors in sexual dysfunction in diabetic female volunteer subjects. Med J Aust. 1989. 151:550–552.25. Tyrer G, Steel JM, Ewing DJ, Bancroft J, Warner P, Clarke BF. Sexual responsiveness in diabetic women. Diabetologia. 1983. 24:166–171.26. Pukall CF, Lahaie MA, Binik YM. Goldstein I, Meston CM, Davis SR, Traish AM, editors. 2006 Sexual Pain Disorders: Pathophysiologic Factors. Women's sexual function and dysfunction- study, diagnosis, and treatment. 2006. 1st ed. London: Taylor & Francis;236–242.27. Kim SW, Kim NN, Jeong SJ, Munarriz R, Goldstein I, Traish AM. Modulation of rat vaginal blood flow and estrogen receptor by estradiol. J Urol. 2004. 172:1538–1543.28. Kim SW, Jeong SJ, Munarriz R, Kim NN, Goldstein I, Traish AM. An in vivo rat model to investigate female vaginal arousal response. J Urol. 2004. 171:1357–1361.29. Pessina MA, Hoyt RF Jr, Goldstein I, Traish AM. Differential regulation of the expression of estrogen, progesterone, and androgen receptors by sex steroid hormones in the vagina: immunohistochemical studies. J Sex Med. 2006. 3:804–814.30. Pessina MA, Hoyt RF Jr, Goldstein I, Traish AM. Differential effects of estradiol, progesterone, and testosterone on vaginal structural integrity. Endocrinology. 2006. 147:61–69.31. Wells CC, Riazi S, Mankhey RW, Bhatti F, Ecelbarger C, Maric C. Diabetic nephropathy is associated with decreased circulating estradiol levels and imbalance in the expression of renal estrogen receptors. Gend Med. 2005. 2:227–237.32. Garris DR, Coleman DL, Morgan CR. Age- and diabetes-related changes in tissue glucose uptake and estradiol accumulation in the C57BL/KsJ mouse. Diabetes. 1985. 34:47–52.33. Garris DR. Diabetes-associated alterations in uterine structure in the C57BL/KsJ mouse: relationship to changes in estradiol accumulation, circulating ovarian steroid levels, and age. Anat Rec. 1985. 211:414–419.34. Park K, Ryu SB, Park YI, Ahn K, Lee SN, Nam JH. Diabetes mellitus induces vaginal tissue fibrosis by TGF-beta 1 expression in the rat model. J Sex Marital Ther. 2001. 27:577–587.35. Park K, Ahn K, Chang JS, Lee SE, Ryu SB, Park YI. Diabetes induced alteration of clitoral hemodynamics and structure in the rabbit. J Urol. 2002. 168:1269–1272.36. Kim NN, Stankovic M, Cushman TT, Goldstein I, Munarriz R, Traish AM. Streptozotocin-induced diabetes in the rat is associated with changes in vaginal hemodynamics, morphology and biochemical markers. BMC Physiol. 2006. 6:4.37. Garris DR, Garris BL. Hypercytolipidemia promotes diabetes (db/db) mutation-associated utero-ovarian involution: counter-regulatory influences of progesterone. Pathophysiology. 2004. 11:41–50.38. Garris DR, Garris BL. Estrogenic restoration of functional pancreatic islet cytoarchitecture in diabetes (db/db) mutant C57BL/KsJ mice: relationship to estradiol localization, systemic glycemia, and persistent hyperinsulinemia. Cell Tissue Res. 2005. 319:231–242.39. Garris DR, Garris BL. Diabetes (db/db) mutation-induced female reproductive tract hypercytolipidemia: estrogenic restoration of utero-ovarian indices. Reprod Toxicol. 2004. 18:641–651.40. Dixon A, Maric C. 17beta-Estradiol attenuates diabetic kidney disease by regulating extracellular matrix and transforming growth factor-beta protein expression and signaling. Am J Physiol Renal Physiol. 2007. 293:F1678–F1790.41. Garris DR. Gonadal steroid modulation of the diabetes (db/db) mutation-induced hyperlipometabolic, hypogonadal syndrome: restoration of female reproductive tract cytochemical and structural indices. Pathophysiology. 2005. 12:109–120.42. Chabrolle C, Jeanpierre E, Tosca L, Ramé C, Dupont J. Effects of high levels of glucose on the steroidogenesis and the expression of adiponectin receptors in rat ovarian cells. Reprod Biol Endocrinol. 2008. 6:11.43. Stamataki KE, Spina J, Rangou DB, Chlouverakis CS, Piaditis GP. Ovarian function in women with non-insulin dependent diabetes mellitus. Clin Endocrinol (Oxf). 1996. 45:615–621.44. La Marca A, Morgante G, Palumbo M, Cianci A, Petraglia F, De Leo V. Insulin-lowering treatment reduces aromatase activity in response to follicle-stimulating hormone in women with polycystic ovary syndrome. Fertil Steril. 2002. 78:1234–1239.45. Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Intern Med. 2002. 137:25–33.46. Ferrini MG, Nolazco G, Vernet D, Gonzalez-Cadavid NF, Berman J. Increased vaginal oxidative stress, apoptosis, and inducible nitric oxide synthase in a diabetic rat model: implications for vaginal fibrosis. Fertil Steril. 2006. 86:1152–1163.47. Cushman T, Kim NN, Hoyt R, Traish A. Estradiol restores diabetes-induced reductions in sex steroid receptor expression and distribution in the vagina of db/db mouse model. J Steroid Biochem Molec Biol. (in press 2009).48. Cai L, Li W, Wang G, Guo L, Jiang Y, Kang YJ. Hyperglycemia-induced apoptosis in mouse myocardium: mitochondrial cytochrome C-mediated caspase-3 activation pathway. Diabetes. 2002. 51:1938–1948.49. Menini S, Iacobini C, Oddi G, Ricci C, Simonelli P, Fallucca S, et al. Increased glomerular cell (podocyte) apoptosis in rats with streptozotocin-induced diabetes mellitus: role in the development of diabetic glomerular disease. Diabetologia. 2007. 50:2591–2599.50. Martin PM, Roon P, Van Ells TK, Ganapathy V, Smith SB. Death of retinal neurons in streptozotocin-induced diabetic mice. Invest Ophthalmol Vis Sci. 2004. 45:3330–3336.51. Gao Q, Gao YM. Hyperglycemic condition disturbs the proliferation and cell death of neural progenitors in mouse embryonic spinal cord. Int J Dev Neurosci. 2007. 25:349–357.52. Gorodeski GI. Estrogen modulation of epithelial permeability in cervical-vaginal cells of premenopausal and postmenopausal women. Menopause. 2007. 14:1012–1019.53. Park K, Han HJ, Kim SW, Jung SI, Kim SO, Lee HS, et al. Expression of aquaporin water channels in rat vagina: potential role in vaginal lubrication. J Sex Med. 2008. 5:77–82.54. Akkus E, Carrier S, Baba K, Hsu GL, Padma-Nathan H, Nunes L, et al. Structural alterations in the tunica albuginea of the penis: impact of Peyronie's disease, ageing and impotence. Br J Urol. 1997. 79:47–53.55. Chin M, Isono M, Isshiki K, Araki S, Sugimoto T, Guo B, et al. Estrogen and raloxifene, a selective estrogen receptor modulator, ameliorate renal damage in db/db mice. Am J Pathol. 2005. 166:1629–1636.56. Kim NN, Min K, Pessina MA, Munarriz R, Goldstein I, Traish AM. Effects of ovariectomy and steroid hormones on vaginal smooth muscle contractility. Int J Impot Res. 2004. 16:43–50.57. Cushman T, Kim NN, Hoyt R, Traish A. Estradiol ameliorates diabetes induced changes in vaginal structure of db/db mouse model. J Sex Med. (in press 2009).58. Giraldi A, Levin RJ. Goldstein I, Meston CM, Davis SR, Traish AM, editors. Vascular Physiology of female sexual function. Women's sexual function and dysfunction-study, diagnosis, and treatment. 2006. 1st ed. London: Taylor & Francis;174–180.59. Giraldi A, Alm P, Werkström V, Myllymäki L, Wagner G, Andersson KE. Morphological and functional characterization of a rat vaginal smooth muscle sphincter. Int J Impot Res. 2002. 14:271–282.60. Traish AM, Kim NN, Munarriz R, Goldstein I. Female genital sexual arousal: biochemical mediators and potential mechanisms of dysfunction. Drug Disc Today. 2004. 1:91–97.61. Giraldi A, Persson K, Werkström V, Alm P, Wagner G, Andersson KE. Effects of diabetes on neurotransmission in rat vaginal smooth muscle. Int J Impot Res. 2001. 13:58–66.62. El-Sakka AI, Lin CS, Chui RM, Dahiya R, Lue TF. Effects of diabetes on nitric oxide synthase and growth factor genes and protein expression in an animal model. Int J Impot Res. 1999. 11:123–132.63. Cellek S, Foxwell NA, Moncada S. Two phases of nitrergic neuropathy in streptozotocin-induced diabetic rats. Diabetes. 2003. 52:2353–2362.64. Caruso S, Rugolo S, Mirabella D, Intelisano G, Di Mari L, Cianci A. Changes in clitoral blood flow in premenopausal women affected by type 1 diabetes after single 100-mg administration of sildenafil. Urology. 2006. 68:161–165.65. Caruso S, Rugolo S, Agnello C, Intelisano G, Di Mari L, Cianci A. Sildenafil improves sexual functioning in premenopausal women with type 1 diabetes who are affected by sexual arousal disorder: a double-blind, crossover, placebo-controlled pilot study. Fertil Steril. 2006. 85:1496–1501.66. Resnick HE, Howard BV. Diabetes and cardiovascular disease. Annu Rev Med. 2002. 53:245–267.67. Masding MG, Stears AJ, Burdge GC, Wootton SA, Sandeman DD. Premenopausal advantages in postprandial lipid metabolism are lost in women with type 2 diabetes. Diabetes Care. 2003. 26:3243–3249.68. Lee SJ, Lee DW, Kim KS, Lee IK. Effect of estrogen on endothelial dysfunction in postmenopausal women with diabetes. Diabetes Res Clin Pract. 2001. 54:Suppl 2. S81–S92.69. Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. J Am Soc Nephrol. 2000. 11:319–329.70. U.S. Renal Data System. USRDS 2000 Annual Data Report. 2000. Bethesda, MD: NIDDK, The National Institutes of Health.71. Mankhey RW, Bhatti F, Maric C. 17beta-Estradiol replacement improves renal function and pathology associated with diabetic nephropathy. Am J Physiol Renal Physiol. 2005. 288:F399–F405.72. Rossi R, Origliani G, Modena MG. Transdermal 17-beta-estradiol and risk of developing type 2 diabetes in a population of healthy, nonobese postmenopausal women. Diabetes Care. 2004. 27:645–649.73. Kernohan AF, Sattar N, Hilditch T, Cleland SJ, Small M, Lumsden MA, et al. Effects of low-dose continuous combined hormone replacement therapy on glucose homeostasis and markers of cardiovascular risk in women with type 2 diabetes. Clin Endocrinol (Oxf). 2007. 66:27–34.74. Berman JR, McCarthy MM, Kyprianou N. Effect of estrogen withdrawal on nitric oxide synthase expression and apoptosis in the rat vagina. Urology. 1998. 51:650–656.75. Rosenfeld CR, Chen C, Roy T, Liu X. Estrogen selectively up-regulates eNOS and nNOS in reproductive arteries by transcriptional mechanisms. J Soc Gynecol Investig. 2003. 10:205–215.76. Ekka E, Vanderheyden I, Glorieux B, de Hertogh R. Estradiol-induced progesterone receptor synthesis in normal and diabetic ovariectomized rat uterus. J Steroid Biochem. 1987. 28:61–64.77. Leavitt WW, Chen TJ, Allen TC. Regulation of progesterone receptor formation by estrogen action. Ann N Y Acad Sci. 1977. 286:210–225.78. Kurita T, Lee KJ, Cooke PS, Taylor JA, Lubahn DB, Cunha GR. Paracrine regulation of epithelial progesterone receptor by estradiol in the mouse female reproductive tract. Biol Reprod. 2000. 62:821–830.79. Pelletier G, Luu-The V, Li S, Labrie F. 2004 localization and estrogenic regulation of androgen receptor mRNA expression in the mouse uterus and vagina. J Endocrinol. 2004. 180:77–85.80. Traish AM, Kim SW, Stankovic M, Goldstein I, Kim NN. Testosterone increases blood flow and expression of androgen and estrogen receptors in the rat vagina. J Sex Med. 2007. 4:609–619.81. Traish AM, Kim N, Min K, Munarriz R, Goldstein I. Role of androgens in female genital sexual arousal: Receptor expression, structure, and function. Fertil Steril. 2002. 77:Suppl 4. S11–S18.82. Alonso A, Fernández R, Moreno M, Ordóñez P, González-Pardo H, Conejo NM, et al. Positive effects of 17beta-estradiol on insulin sensitivity in aged ovariectomized female rats. J Gerontol A Biol Sci Med Sci. 2006. 61:419–426.83. Andersson B, Mattsson LA, Hahn L, Mårin P, Lapidus L, Holm G, et al. Estrogen replacement therapy decreases hyperandrogenicity and improves glucose homeostasis and plasma lipids in postmenopausal women with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1997. 82:638–643.84. Araújo DA, Farias ML, Andrade AT. Effects of transdermal and oral estrogen replacement on lipids and glucose metabolism in postmenopausal women with type 2 diabetes mellitus. Climacteric. 2002. 5:286–292.85. Cagnacci A, Tuveri F, Cirillo R, Setteneri AM, Melis GB, Volpe A. The effect of transdermal 17-beta-estradiol on glucose metabolism of postmenopausal women is evident during the oral but not the intravenous glucose administration. Maturitas. 1997. 28:163–167.86. Barros RP, Machado UF, Warner M, Gustafsson JA. Muscle GLUT4 regulation by estrogen receptors ERbeta and ERalpha. Proc Natl Acad Sci U S A. 2006. 103:1605–1608.87. Barros RP, Machado UF, Gustafsson JA. Estrogen receptors: new players in diabetes mellitus. Trends Mol Med. 2006. 12:425–431.88. Cheng CM, Cohen M, Wang J, Bondy CA. Estrogen augments glucose transporter and IGF1 expression in primate cerebral cortex. FASEB J. 2001. 15:907–915.89. Mankhey RW, Wells CC, Bhatti F, Maric C. 17beta-estradiol supplementation reduces tubulointerstitial fibrosis by increasing MMP activity in the diabetic kidney. Am J Physiol Regul Integr Comp Physiol. 2007. 292:R769–R777.90. Shim GJ, Kis LL, Warner M, Gustafsson JA. Autoimmune glomerulonephritis with spontaneous formation of splenic germinal centers in mice lacking the estrogen receptor alpha gene. Proc Natl Acad Sci U S A. 2004. 101:1720–1724.91. Traish AM, Kim NN, Huang YH, Min K, Munarriz R, Goldstein I. Sex steroid hormones differentially regulate nitric oxide synthase and arginase activities in the proximal and distal rabbit vagina. Int J Impot Res. 2003. 15:397–404.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Medical Treatment of the Female Sexual Dysfunction
- Predictors of Sexual Desire, Arousal, Lubrication, Orgasm, Satisfaction, and Pain in Women with Gynecologic Cancer
- Laser Doppler Flowmetry: Usefulness in Female Sexual Dysfunction
- Models of Sexual Response in Humans
- Pharmacologic management of female sexual dysfunction