J Breast Cancer.
2016 Jun;19(2):156-162. 10.4048/jbc.2016.19.2.156.
High Expression of Urokinase-Type Plasminogen Activator Is Associated with Lymph Node Metastasis of Invasive Ductal Carcinoma of the Breast
- Affiliations
-
- 1Department of Surgery, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea. chanheun1@gmail.com
- 2Department of Pathology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2308966
- DOI: http://doi.org/10.4048/jbc.2016.19.2.156
Abstract
- PURPOSE
In the present study, we evaluated the levels of urokinase-type plasminogen activator (uPA) and plasminogen activator inhibitor 1 (PAI-1) by performing immunohistochemical staining to determine whether they were reliable prognostic markers in patients with breast cancer.
METHODS
Demographic and clinicopathological parameters of 214 patients with invasive ductal carcinoma (IDC) and 80 patients with ductal carcinoma in situ (DCIS) who were diagnosed and treated from 2006 to 2010 were analyzed. Tissue microarray was constructed and immunohistochemical staining was performed for each specimen.
RESULTS
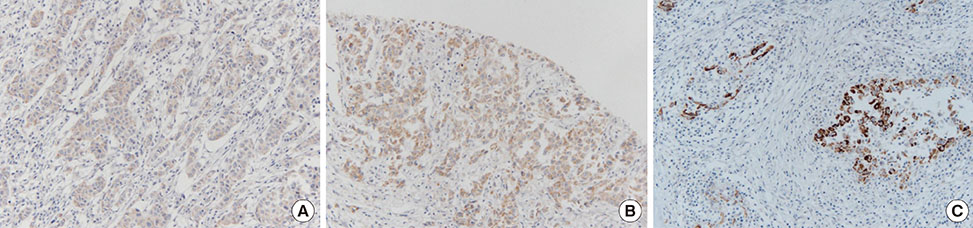
Univariate analyses showed that age at diagnosis, history of hormone replacement therapy, radiation therapy, skin and chest wall invasion, Paget disease, lymphovascular invasion, estrogen receptor positivity, and triple-negative subtype were significantly associated with patient prognosis (p<0.005). Patients with DCIS showed higher PAI-1 expression than patients with IDC (82.5% and 36.2%, respectively; p=0.012). Lymph node metastasis was more frequent in patients with high uPA levels than in patients with low uPA levels (p=0.001).
CONCLUSION
Our results suggested that PAI-1 was involved in tumor progression in the early stages of breast cancer, such as DCIS. In addition, our results suggested that high uPA levels were associated with the lymph node metastasis of IDC.
MeSH Terms
-
Breast Neoplasms
Breast*
Carcinoma, Ductal*
Carcinoma, Intraductal, Noninfiltrating
Diagnosis
Estrogens
Hormone Replacement Therapy
Humans
Lymph Nodes*
Neoplasm Metastasis*
Plasminogen Activator Inhibitor 1
Prognosis
Skin
Thoracic Wall
Urokinase-Type Plasminogen Activator*
Estrogens
Plasminogen Activator Inhibitor 1
Urokinase-Type Plasminogen Activator
Figure
Reference
-
1. Isaacs C, Stearns V, Hayes DF. New prognostic factors for breast cancer recurrence. Semin Oncol. 2001; 28:53–67.
Article2. Duffy MJ, Duggan C. The urokinase plasminogen activator system: a rich source of tumour markers for the individualised management of patients with cancer. Clin Biochem. 2004; 37:541–548.
Article3. Duffy MJ, O'Grady P, Devaney D, O'Siorain L, Fennelly JJ, Lijnen HJ. Urokinase-plasminogen activator, a marker for aggressive breast carcinomas: preliminary report. Cancer. 1988; 62:531–533.
Article4. Jänicke F, Schmitt M, Graeff H. Clinical relevance of the urokinase-type and tissue-type plasminogen activators and of their type 1 inhibitor in breast cancer. Semin Thromb Hemost. 1991; 17:303–312.
Article5. Hansen S, Overgaard J, Rose C, Knoop A, Laenkholm AV, Andersen J, et al. Independent prognostic value of angiogenesis and the level of plasminogen activator inhibitor type 1 in breast cancer patients. Br J Cancer. 2003; 88:102–108.
Article6. Jänicke F, Prechtl A, Thomssen C, Harbeck N, Meisner C, Untch M, et al. Randomized adjuvant chemotherapy trial in high-risk, lymph nodenegative breast cancer patients identified by urokinase-type plasminogen activator and plasminogen activator inhibitor type 1. J Natl Cancer Inst. 2001; 93:913–920.
Article7. Harbeck N, Schmitt M, Meisner C, Friedel C, Untch M, Schmidt M, et al. Ten-year analysis of the prospective multicentre Chemo-N0 trial validates American Society of Clinical Oncology (ASCO)-recommended biomarkers uPA and PAI-1 for therapy decision making in node-negative breast cancer patients. Eur J Cancer. 2013; 49:1825–1835.
Article8. Schmitt M, Harbeck N, Brünner N, Jänicke F, Meisner C, Mühlenweg B, et al. Cancer therapy trials employing level-of-evidence-1 disease forecast cancer biomarkers uPA and its inhibitor PAI-1. Expert Rev Mol Diagn. 2011; 11:617–634.
Article9. Kantelhardt EJ, Vetter M, Schmidt M, Veyret C, Augustin D, Hanf V, et al. Prospective evaluation of prognostic factors uPA/PAI-1 in nodenegative breast cancer: phase III NNBC3-Europe trial (AGO, GBG, EORTC-PBG) comparing 6×FEC versus 3×FEC/3×Docetaxel. BMC Cancer. 2011; 11:140.10. Saadoun H, Lamy PJ, Thezenas S, Pouderoux S, Bibeau F, Montels F, et al. Prognostic impact of the inclusion of uPA/PAI-1 tumor levels in the current adjuvant treatment decision-making for early breast cancer. Future Oncol. 2014; 10:195–209.
Article11. Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe. 1987; 8:138–140.12. Duffy MJ. Proteases as prognostic markers in cancer. Clin Cancer Res. 1996; 2:613–618.13. Lamy PJ, Verjat T, Servanton AC, Paye M, Leissner P, Mougin B. Urokinase-type plasminogen activator and plasminogen activator inhibitor type-1 mRNA assessment in breast cancer by means of NASBA: correlation with protein expression. Am J Clin Pathol. 2007; 128:404–413.
Article14. Dass K, Ahmad A, Azmi AS, Sarkar SH, Sarkar FH. Evolving role of uPA/uPAR system in human cancers. Cancer Treat Rev. 2008; 34:122–136.
Article15. Tang L, Han X. The urokinase plasminogen activator system in breast cancer invasion and metastasis. Biomed Pharmacother. 2013; 67:179–182.
Article16. Roca C, Primo L, Valdembri D, Cividalli A, Declerck P, Carmeliet P, et al. Hyperthermia inhibits angiogenesis by a plasminogen activator inhibitor 1-dependent mechanism. Cancer Res. 2003; 63:1500–1507.17. Soff GA, Sanderowitz J, Gately S, Verrusio E, Weiss I, Brem S, et al. Expression of plasminogen activator inhibitor type 1 by human prostate carcinoma cells inhibits primary tumor growth, tumor-associated angiogenesis, and metastasis to lung and liver in an athymic mouse model. J Clin Invest. 1995; 96:2593–2600.
Article18. Bajou K, Maillard C, Jost M, Lijnen RH, Gils A, Declerck P, et al. Hostderived plasminogen activator inhibitor-1 (PAI-1) concentration is critical for in vivo tumoral angiogenesis and growth. Oncogene. 2004; 23:6986–6990.
Article19. Leik CE, Su EJ, Nambi P, Crandall DL, Lawrence DA. Effect of pharmacologic plasminogen activator inhibitor-1 inhibition on cell motility and tumor angiogenesis. J Thromb Haemost. 2006; 4:2710–2715.
Article20. Castelló R, España F, Vázquez C, Fuster C, Almenar SM, Aznar J, et al. Plasminogen activator inhibitor-1 4G/5G polymorphism in breast cancer patients and its association with tissue PAI-1 levels and tumor severity. Thromb Res. 2006; 117:487–492.
Article21. Lei H, Hemminki K, Johansson R, Altieri A, Enquist K, Henriksson R, et al. PAI-1 -675 4G/5G polymorphism as a prognostic biomarker in breast cancer. Breast Cancer Res Treat. 2008; 109:165–175.
Article22. Malinowsky K, Wolff C, Berg D, Schuster T, Walch A, Bronger H, et al. uPA and PAI-1-related signaling pathways differ between primary breast cancers and lymph node metastases. Transl Oncol. 2012; 5:98–104.
Article23. Look MP, van Putten WL, Duffy MJ, Harbeck N, Christensen IJ, Thomssen C, et al. Pooled analysis of prognostic impact of urokinasetype plasminogen activator and its inhibitor PAI-1 in 8377 breast cancer patients. J Natl Cancer Inst. 2002; 94:116–128.
Article24. Duffy MJ, McGowan PM, Harbeck N, Thomssen C, Schmitt M. uPA and PAI-1 as biomarkers in breast cancer: validated for clinical use in level-of-evidence-1 studies. Breast Cancer Res. 2014; 16:428.
Article25. Andres SA, Edwards AB, Wittliff JL. Expression of urokinase-type plasminogen activator (uPA), its receptor (uPAR), and inhibitor (PAI-1) in human breast carcinomas and their clinical relevance. J Clin Lab Anal. 2012; 26:93–103.
Article26. Ferrier CM, de Witte HH, Straatman H, van Tienoven DH, van Geloof WL, Rietveld FJ, et al. Comparison of immunohistochemistry with immunoassay (ELISA) for the detection of components of the plasminogen activation system in human tumour tissue. Br J Cancer. 1999; 79:1534–1541.
Article27. Christensen L, Wiborg Simonsen AC, Heegaard CW, Moestrup SK, Andersen JA, Andreasen PA. Immunohistochemical localization of urokinase-type plasminogen activator, type-1 plasminogen-activator inhibitor, urokinase receptor and alpha(2)-macroglobulin receptor in human breast carcinomas. Int J Cancer. 1996; 66:441–452.
Article28. Haas S, Park TW, Hahne JC, Fischer HP. Influence of preoperative core biopsies on uPA/PAI-1 expression in breast cancer tissue. Virchows Arch. 2008; 452:277–283.
Article29. Lang DS, Heilenkötter U, Schumm W, Behrens O, Simon R, Vollmer E, et al. Optimized immunohistochemistry in combination with image analysis: a reliable alternative to quantitative ELISA determination of uPA and PAI-1 for routine risk group discrimination in breast cancer. Breast. 2013; 22:736–743.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Urokinase-type Plasminogen Activator (uPA) and Plasminogen Activator Inhibitor-1 (PAI-1) in Gallbladder Carcinoma
- Urokinase-type plasminogen activator receptor in IgA nephropathy
- Expression of Matrix-Metalloproteinase-2, Urokinase-Type Plasminogen Activator and Plasminogen Activator Inhibitor-1 in Invasion Mode and Lymph Node Metastasis of Laryngeal Squamous Cell Carcinoma
- The Relationship between Plasminogen Acti- vator Inhibitor-1 and Bone Marrow Microme- tastases in Breast Cancer
- Comparison of the Expression of Variants of CD44 between Node-positive and Node-negative Breast Carcinomas