Anat Cell Biol.
2016 Jun;49(2):88-98. 10.5115/acb.2016.49.2.88.
Vitamin C is taken up by human T cells via sodium-dependent vitamin C transporter 2 (SVCT2) and exerts inhibitory effects on the activation of these cells in vitro
- Affiliations
-
- 1Department of Anatomy and Cell Biology, Seoul National University College of Medicine, Seoul, Korea. hyi830@snu.ac.kr
- KMID: 2308915
- DOI: http://doi.org/10.5115/acb.2016.49.2.88
Abstract
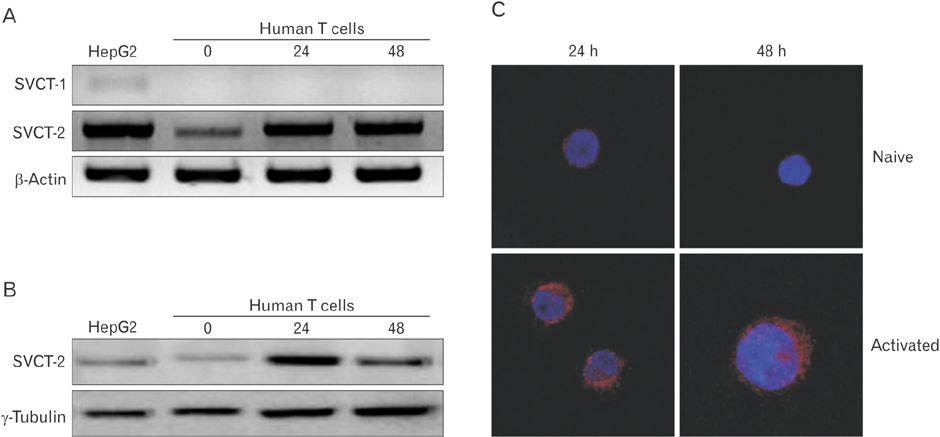
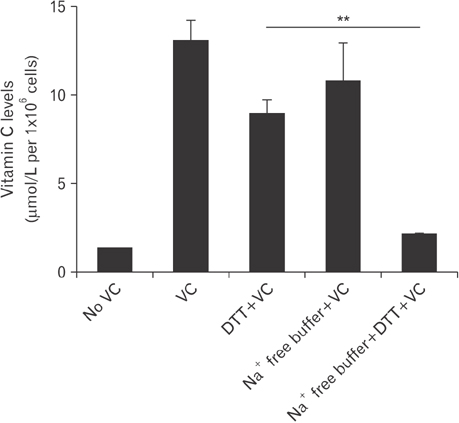
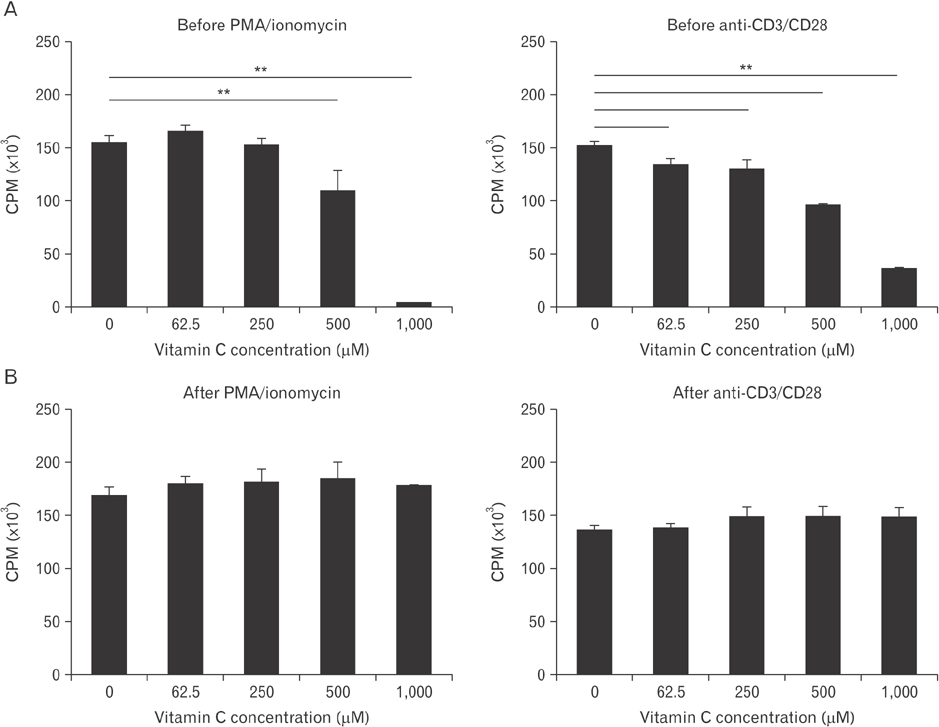
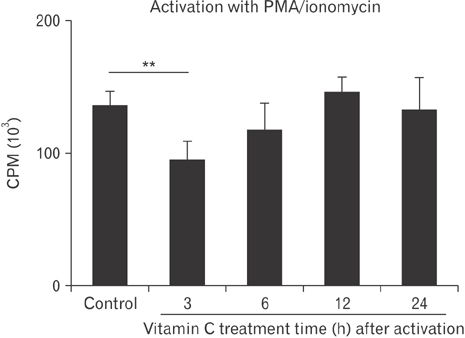
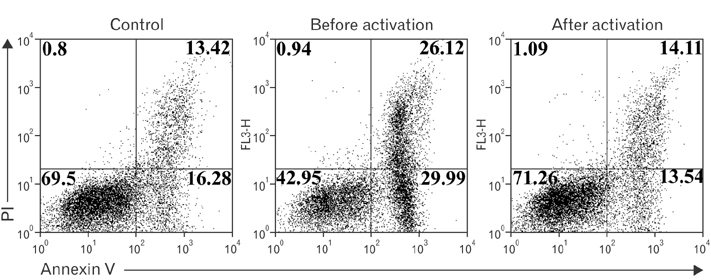
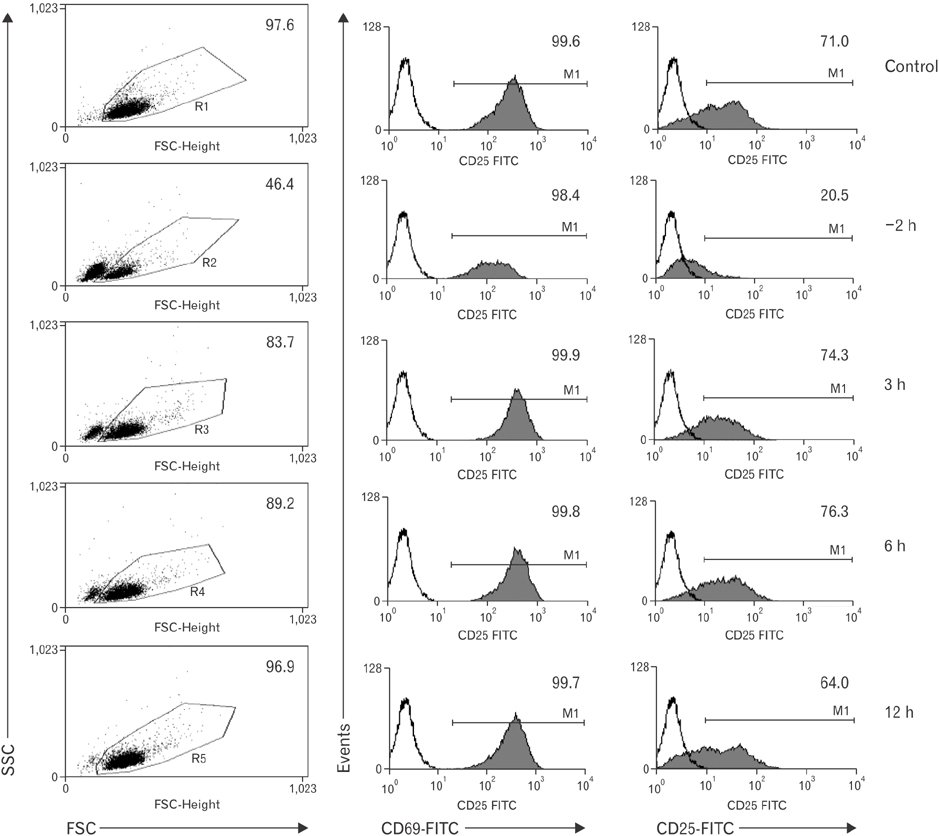
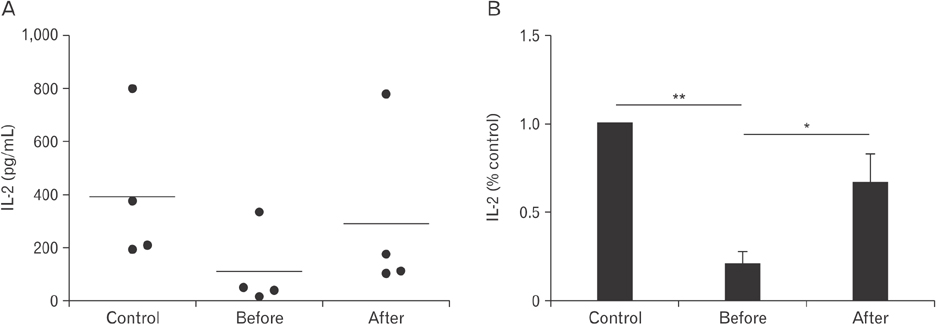
- Vitamin C is an essential micronutrient that affects immune responses. T cells are one of the main players in acquired immunity and have been reported to be influenced by in vivo vitamin C supplementation. Yet, the way by which T cells uptake vitamin C and what direct effects vitamin C exerts on the cells are not known. To elucidate, we isolated human peripheral blood T cells and analyzed the expression of sodium-dependent vitamin C transporters (SVCT). T cells were activated in vitro in the absence or presence of vitamin C, before or after activation. As results, human T cells expressed SVCT2, but not SVCT1, and the expression level increased following activation. Vitamin C added in the culture media generally did not affect T-cell behaviors following activation, such as proliferation, apoptosis, expression of CD25 and CD69, and interleukin 2 secretion, regardless whether it was added before or after activation. However, exceptionally, high concentration vitamin C, when it was added before activation, but not after activation, did exert toxic effects on cell activation with respect to the above-mentioned parameters. In conclusion, we showed the expression of SVCT2 in human T cells for the first time. Vitamin C exerted toxic effects, at least in vitro, when the concentration was high and when it was given before activation. These toxic effects are not thought to be via anti-oxidant effects of vitamin C.
Keyword
MeSH Terms
Figure
Reference
-
1. Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989; 86:6377–6381.2. Rumsey SC, Kwon O, Xu GW, Burant CF, Simpson I, Levine M. Glucose transporter isoforms GLUT1 and GLUT3 transport dehydroascorbic acid. J Biol Chem. 1997; 272:18982–18989.3. Vera JC, Rivas CI, Fischbarg J, Golde DW. Mammalian facilitative hexose transporters mediate the transport of dehydroascorbic acid. Nature. 1993; 364:79–82.4. Faaland CA, Race JE, Ricken G, Warner FJ, Williams WJ, Holtzman EJ. Molecular characterization of two novel transporters from human and mouse kidney and from LLC-PK1 cells reveals a novel conserved family that is homologous to bacterial and Aspergillus nucleobase transporters. Biochim Biophys Acta. 1998; 1442:353–360.5. Daruwala R, Song J, Koh WS, Rumsey SC, Levine M. Cloning and functional characterization of the human sodium-dependent vitamin C transporters hSVCT1 and hSVCT2. FEBS Lett. 1999; 460:480–484.6. Rajan DP, Huang W, Dutta B, Devoe LD, Leibach FH, Ganapathy V, Prasad PD. Human placental sodium-dependent vitamin C transporter (SVCT2): molecular cloning and transport function. Biochem Biophys Res Commun. 1999; 262:762–768.7. Tsukaguchi H, Tokui T, Mackenzie B, Berger UV, Chen XZ, Wang Y, Brubaker RF, Hediger MA. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature. 1999; 399:70–75.8. Nishikimi M, Fukuyama R, Minoshima S, Shimizu N, Yagi K. Cloning and chromosomal mapping of the human nonfunctional gene for L-gulono-gamma-lactone oxidase, the enzyme for L-ascorbic acid biosynthesis missing in man. J Biol Chem. 1994; 269:13685–13688.9. National Institutes of Health. Vitamin C [Internet]. Bethesda, MD: National Institutes of Health;2016. cited 2016 Mar 1. Available from: https://ods.od.nih.gov/factsheets/VitaminCConsumer/.10. Johnston CS. Biomarkers for establishing a tolerable upper intake level for vitamin C. Nutr Rev. 1999; 57:71–77.11. Noh K, Lim H, Moon SK, Kang JS, Lee WJ, Lee D, Hwang YI. Mega-dose vitamin C modulates T cell functions in Balb/c mice only when administered during T cell activation. Immunol Lett. 2005; 98:63–72.12. Prinz W, Bortz R, Bregin B, Hersch M. The effect of ascorbic acid supplementation on some parameters of the human immunological defence system. Int J Vitam Nutr Res. 1977; 47:248–257.13. Feigen GA, Smith BH, Dix CE, Flynn CJ, Peterson NS, Rosenberg LT, Pavlović S, Leibovitz B. Enhancement of antibody production and protection against systemic anaphylaxis by large doses of vitamin C. Res Commun Chem Pathol Pharmacol. 1982; 38:313–333.14. Anderson R, Dittrich OC. Effects of ascorbate on leucocytes: Part IV. Increased neutrophil function and clinical improvement after oral ascorbate in 2 patients with chronic granulomatous disease. S Afr Med J. 1979; 56:476–480.15. Jeong YJ, Hong SW, Kim JH, Jin DH, Kang JS, Lee WJ, Hwang YI. Vitamin C-treated murine bone marrow-derived dendritic cells preferentially drive naive T cells into Th1 cells by increased IL-12 secretions. Cell Immunol. 2011; 266:192–199.16. Kennes B, Dumont I, Brohee D, Hubert C, Neve P. Effect of vitamin C supplements on cell-mediated immunity in old people. Gerontology. 1983; 29:305–310.17. Cetinkale O, Senel O, Bulan R. The effect of antioxidant therapy on cell-mediated immunity following burn injury in an animal model. Burns. 1999; 25:113–118.18. Maeng HG, Lim H, Jeong YJ, Woo A, Kang JS, Lee WJ, Hwang YI. Vitamin C enters mouse T cells as dehydroascorbic acid in vitro and does not recapitulate in vivo vitamin C effects. Immunobiology. 2009; 214:311–320.19. Woo A, Kim JH, Jeong YJ, Maeng HG, Lee YT, Kang JS, Lee WJ, Hwang YI. Vitamin C acts indirectly to modulate isotype switching in mouse B cells. Anat Cell Biol. 2010; 43:25–35.20. Jeong YJ, Kim JH, Hong JM, Kang JS, Kim HR, Lee WJ, Hwang YI. Vitamin C treatment of mouse bone marrow-derived dendritic cells enhanced CD8(+) memory T cell production capacity of these cells in vivo. Immunobiology. 2014; 219:554–564.21. Anderson R, Oosthuizen R, Maritz R, Theron A, Van Rensburg AJ. The effects of increasing weekly doses of ascorbate on certain cellular and humoral immune functions in normal volunteers. Am J Clin Nutr. 1980; 33:71–76.22. VanderJagt DJ, Garry PJ, Bhagavan HN. Ascorbate and dehydroascorbate: distribution in mononuclear cells of healthy elderly people. Am J Clin Nutr. 1989; 49:511–516.23. Williams MS, Kwon J. T cell receptor stimulation, reactive oxygen species, and cell signaling. Free Radic Biol Med. 2004; 37:1144–1151.24. Belikov AV, Schraven B, Simeoni L. T cells and reactive oxygen species. J Biomed Sci. 2015; 22:85.25. García Mde L, Salazar K, Millán C, Rodríguez F, Montecinos H, Caprile T, Silva C, Cortes C, Reinicke K, Vera JC, Aguayo LG, Olate J, Molina B, Nualart F. Sodium vitamin C cotransporter SVCT2 is expressed in hypothalamic glial cells. Glia. 2005; 50:32–47.26. Seno T, Inoue N, Matsui K, Ejiri J, Hirata K, Kawashima S, Yokoyama M. Functional expression of sodium-dependent vitamin C transporter 2 in human endothelial cells. J Vasc Res. 2004; 41:345–351.27. Nualart FJ, Rivas CI, Montecinos VP, Godoy AS, Guaiquil VH, Golde DW, Vera JC. Recycling of vitamin C by a bystander effect. J Biol Chem. 2003; 278:10128–10133.28. Wang Y, Mackenzie B, Tsukaguchi H, Weremowicz S, Morton CC, Hediger MA. Human vitamin C (L-ascorbic acid) transporter SVCT1. Biochem Biophys Res Commun. 2000; 267:488–494.29. Takanaga H, Mackenzie B, Hediger MA. Sodium-dependent ascorbic acid transporter family SLC23. Pflugers Arch. 2004; 447:677–682.30. Reidling JC, Subramanian VS, Dahhan T, Sadat M, Said HM. Mechanisms and regulation of vitamin C uptake: studies of the hSVCT systems in human liver epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2008; 295:G1217–G1227.31. Clement MV, Ramalingam J, Long LH, Halliwell B. The in vitro cytotoxicity of ascorbate depends on the culture medium used to perform the assay and involves hydrogen peroxide. Antioxid Redox Signal. 2001; 3:157–163.32. Berger J, Shepard D, Morrow F, Taylor A. Relationship between dietary intake and tissue levels of reduced and total vitamin C in the nonscorbutic guinea pig. J Nutr. 1989; 119:734–740.33. Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008; 26:453–479.34. Savini I, Rossi A, Pierro C, Avigliano L, Catani MV. SVCT1 and SVCT2: key proteins for vitamin C uptake. Amino Acids. 2008; 34:347–355.35. Amatya R, Vajpayee M, Kaushik S, Kanswal S, Pandey RM, Seth P. Lymphocyte immunophenotype reference ranges in healthy Indian adults: implications for management of HIV/AIDS in India. Clin Immunol. 2004; 112:290–295.36. Jacob RA, Pianalto FS, Agee RE. Cellular ascorbate depletion in healthy men. J Nutr. 1992; 122:1111–1118.37. Eylar E, Báez I, Navas J, Mercado C. Sustained levels of ascorbic acid are toxic and immunosuppressive for human T cells. P R Health Sci J. 1996; 15:21–26.38. Härtel C, Strunk T, Bucsky P, Schultz C. Effects of vitamin C on intracytoplasmic cytokine production in human whole blood monocytes and lymphocytes. Cytokine. 2004; 27:101–106.39. Tatla S, Woodhead V, Foreman JC, Chain BM. The role of reactive oxygen species in triggering proliferation and IL-2 secretion in T cells. Free Radic Biol Med. 1999; 26:14–24.40. Kamiński MM, Röth D, Sass S, Sauer SW, Krammer PH, Gülow K. Manganese superoxide dismutase: a regulator of T cell activation-induced oxidative signaling and cell death. Biochim Biophys Acta. 2012; 1823:1041–1052.41. Murphy MP, Siegel RM. Mitochondrial ROS fire up T cell activation. Immunity. 2013; 38:201–202.42. Chaudhri G, Hunt NH, Clark IA, Ceredig R. Antioxidants inhibit proliferation and cell surface expression of receptors for interleukin-2 and transferrin in T lymphocytes stimulated with phorbol myristate acetate and ionomycin. Cell Immunol. 1988; 115:204–213.43. Dornand J, Gerber M. Inhibition of murine T-cell responses by anti-oxidants: the targets of lipo-oxygenase pathway inhibitors. Immunology. 1989; 68:384–391.44. Kaminski MM, Sauer SW, Klemke CD, Süss D, Okun JG, Krammer PH, Gülow K. Mitochondrial reactive oxygen species control T cell activation by regulating IL-2 and IL-4 expression: mechanism of ciprofloxacin-mediated immunosuppression. J Immunol. 2010; 184:4827–4841.45. Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000; 348 Pt 3:607–614.46. Podmore ID, Griffiths HR, Herbert KE, Mistry N, Mistry P, Lunec J. Vitamin C exhibits pro-oxidant properties. Nature. 1998; 392:559.47. Hildeman DA. Regulation of T-cell apoptosis by reactive oxygen species. Free Radic Biol Med. 2004; 36:1496–1504.48. Bauer MK, Vogt M, Los M, Siegel J, Wesselborg S, Schulze-Osthoff K. Role of reactive oxygen intermediates in activationinduced CD95 (APO-1/Fas) ligand expression. J Biol Chem. 1998; 273:8048–8055.49. Sandstrom PA, Mannie MD, Buttke TM. Inhibition of activationinduced death in T cell hybridomas by thiol antioxidants: oxidative stress as a mediator of apoptosis. J Leukoc Biol. 1994; 55:221–226.50. Nathan C, Cunningham-Bussel A. Beyond oxidative stress: an immunologist's guide to reactive oxygen species. Nat Rev Immunol. 2013; 13:349–361.51. Chou PT, Khan AU. L-ascorbic acid quenching of singlet delta molecular oxygen in aqueous media: generalized antioxidant property of vitamin C. Biochem Biophys Res Commun. 1983; 115:932–937.52. Kamiński MM, Röth D, Krammer PH, Gülow K. Mitochondria as oxidative signaling organelles in T-cell activation: physiological role and pathological implications. Arch Immunol Ther Exp (Warsz). 2013; 61:367–384.53. Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA, Wang CR, Schumacker PT, Licht JD, Perlman H, Bryce PJ, Chandel NS. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 2013; 38:225–236.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Long-term tumor suppression in cholangiocarcinoma using cytokine-induced killer cell therapy and high-dose vitamin C: a case report
- Vitamin D and Immune Responses
- Expression of Sodium-dependent Vitamin C Transporter in Rat Dermal Fibroblasts
- Immunohistochemical Study on the SVCT Expression in the Spinal Cord of Experimental Allergic Encephalomyelitis (EAE) Model
- The Role of Vitamin D in Adipose Tissue Biology: Adipocyte Differentiation, Energy Metabolism, and Inflammation