Korean Diabetes J.
2008 Oct;32(5):409-417. 10.4093/kdj.2008.32.5.409.
Association of Serum Cystatin C with Metabolic Syndrome and Its Related Components in Korean Adults
- Affiliations
-
- 1Department of Internal Medicine, School of Medicine, Pusan National University, Korea.
- KMID: 2298079
- DOI: http://doi.org/10.4093/kdj.2008.32.5.409
Abstract
- BACKGROUND
Serum cystatin C has been reported as a better marker than serum creatinine for estimation of kidney function and may be associated with cardiovascular disease. The aim of this study was to elucidate the association of serum cystatin C with metabolic syndrome (MS), a constellation of cardiovascular risk factors, and its related components and the usefulness of serum cystatin C for the cardiovascular risk assessment.
METHODS
1,468 healthy subjects (814 men and 655 women), who visited health promotion center of Pusan National University Hospital for routine medical checkup were included. MS was defined by modified, revised National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP) III criteria.
RESULTS
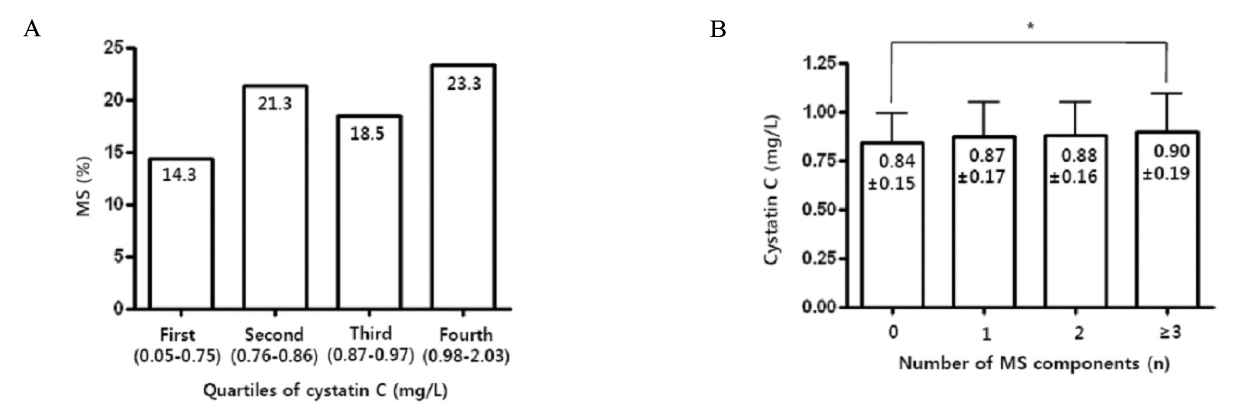
Mean serum cystatin C value was 0.87 +/- 0.17 mg/L. In partial correlation analysis adjusted by age, sex and Glomerular Filtration Rate (GFR), cystatin C was associated with most of metabolic parameters and especially had significant positive correlation with waist circumference (r = 0.215), triglyceride (TG) (r = 0.141), diastolic blood pressure (BP) (r = 0.116), and correlated negatively with high density lipoprotein (HDL) cholesterol (r = -0.152) (all P < 0.001). There were increasing trends of prevalence of MS with the increase of quartiles of cystatin C and as the number of MS components increased, cystatin C values significantly increased. Serum cystatin C was also significantly increased in MS (0.90 +/- 0.19 mg/L vs. 0.86 +/- 0.16 mg/L). In stepwise multiple regression analysis including the components of MS, Waist circumference, diastolic BP, triglyceride, and HDL cholesterol were independent determinants of serum cystatin C, but with creatinine, only waist circumference was independent determinant.
CONCLUSIONS
Serum cystatin C was closely associated with MS and its related cardiovascular risk factors and might be useful as a tool of cardiovascular risk assessment.
MeSH Terms
Figure
Reference
-
1. Tanaka A, Suemaru K, Araki H. A new approach for evaluating renal function and its practical application. J Pharmacol Sci. 2007. 105:1–5.2. Laterza OF, Price CP, Scott MG. Cystatin C: an improved estimator of glomerular filtration rate? Clin Chem. 2002. 48:699–707.3. Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002. 40:221–226.4. Tanaka A, Suemaru K, Otsuka T, Ido K, Nishimiya T, Sakai I, Hasegawa H, Inoue T, Murase M, Yasukawa M, Araki H. Estimation of the initial dose setting of vancomycin therapy with use of cystatin C as a new marker of renal function. Ther Drug Monit. 2007. 29:261–264.5. Larsson A, Helmersson J, Hansson LO, Basu S. Serum cystatin C is associated with other cardiovascular risk markers and cardiovascular disease in elderly men. Int J Cardiol. 2008. 125:263–264.6. Ogawa Y, Goto T, Tamasawa N, Matsui J, Tando Y, Sugimoto K, Tomotsune K, Kimura M, Yasujima M, Suda T. Serum cystatin C in diabetic patients. Not only an indicator for renal dysfunction in patients with overt nephropathy but also a predictor for cardiovascular events in patients without nephropathy. Diabetes Res Clin Pract. 2008. 79:357–361.7. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, Spertus JA, Costa F. American Heart Association; National Heart, Lung, and Blood Institute: Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005. 112:2735–2752.8. Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002. 288:2709–2716.9. Malik S, Wong ND, Franklin SS, Kamath TV, L'Italien GJ, Pio JR, Williams GR. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004. 110:1245–1250.10. Chen J, Muntner P, Hamm LL, Jones DW, Batuman V, Fonseca V, Whelton PK, He J. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med. 2004. 140:167–174.11. Chen J, Gu D, Chen CS, Wu X, Hamm LL, Muntner P, Batuman V, Lee CH, Whelton PK, He J. Association between the metabolic syndrome and chronic kidney disease in Chinese adults. Nephrol Dial Transplant. 2007. 22:1100–1106.12. Watanabe S, Okura T, Liu J, Miyoshi K, Fukuoka T, Hiwada K, Higaki J. Serum cystatin C level is a marker of end-organ damage in patients with essential hypertension. Hypertens Res. 2003. 26:895–899.13. Rodilla E, Costa JA, Pérez Lahiguera F, González C, Miralles A, Pascual JM. Cystatin C and other cardiovascular markers in hypertension. Med Clin (Barc). 2008. 130:1–5.14. Lee BW, Ihm SH, Choi MG, Yoo HJ. The comparison of cystatin C and creatinine as an accurate serum marker in the prediction of type 2 diabetic nephropathy. Diabetes Res Clin Pract. 2007. 78:428–434.15. Kang YH, Min HK, Son SM, Kim IJ, Kim YK. The association of serum gamma glutamyltransferase with components of the metabolic syndrome in the Korean adults. Diabetes Res Clin Pract. 2007. 77:306–313.16. Levy AS, Greene T, Kusek J, Beck GJ, Group MS. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol. 2000. 11:A0828. (abstract).17. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004. 363:157–163.18. Finney H, Newman DJ, Price CP. Adult reference ranges for serum cystatin C, creatinine and predicted creatinine clearance. Ann Clin Biochem. 2000. 37(Pt 1):49–59.19. Finney H, Newman DJ, Thakkar H, Fell JM, Price CP. Reference ranges for plasma cystatin C and creatinine measurements in premature infants, neonates, and older children. Arch Dis Child. 2000. 82:71–75.20. Vinge E, Lindergard B, Nilsson-Ehle P, Grubb A. Relationships among serum cystatin C, serum creatinine, lean tissue mass and glomerular filtration rate in healthy adults. Scand J Clin Lab Invest. 1999. 59:587–592.21. Sjöström P, Tidman M, Jones I. Determination of the production rate and non-renal clearance of cystatin C and estimation of the glomerular filtration rate from the serum concentration of cystatin C in humans. Scand J Clin Lab Invest. 2005. 65:111–124.22. Norlund L, Fex G, Lanke J, Von Schenck H, Nilsson JE, Leksell H, Grubb A. Reference intervals for the glomerular filtration rate and cell-proliferation markers: serum cystatin C and serum beta 2-microglobulin/cystatin C-ratio. Scand J Clin Lab Invest. 1997. 57:463–470.23. Knight EL, Verhave JC, Spiegelman D, Hillege HL, de Zeeuw D, Curhan GC, de Jong PE. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004. 65:1416–1421.24. Köttgen A, Selvin E, Stevens LA, Levey AS, Van Lente F, Coresh J. Serum cystatin C in the United States: the Third National Health and Nutrition Examination Survey (NHANES III). Am J Kidney Dis. 2008. 51:385–394.25. Retnakaran R, Connelly PW, Harris SB, Zinman B, Hanley AJ. Cystatin C is associated with cardiovascular risk factors and metabolic syndrome in Aboriginal youth. Pediatr Nephrol. 2007. 22:1007–1013.26. Lee DS, Evans JC, Robins SJ, Wilson PW, Albano I, Fox CS, Wang TJ, Benjamin EJ, D'Agostino RB, Vasan RS. Gamma glutamyltransferase and metabolic syndrome, cardiovascular disease, and mortality risk: the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2007. 27:127–133.27. Servais A, Giral P, Bernard M, Bruckert E, Deray G, Isnard Bagnis C. Is serum cystatin-C a reliable marker for metabolic syndrome? Am J Med. 2008. 121:426–432.28. Iseki K, Ikemiya Y, Kinjo K, Inoue T, Iseki C, Takishita S. Body mass index and the risk of development of end-stage renal disease in a screened cohort. Kidney Int. 2004. 65:1870–1876.29. Muntner P, Coresh J, Smith JC, Eckfeldt J, Klag MJ. Plasma lipids and risk of developing renal dysfunction: the atherosclerosis risk in communities study. Kidney Int. 2000. 58:293–301.30. Sechi LA, Catena C, Zingaro L, Melis A, De Marchi S. Abnormalities of glucose metabolism in patients with early renal failure. Diabetes. 2002. 51:1226–1232.31. Chen J, Muntner P, Hamm LL, Fonseca V, Batuman V, Whelton PK, He J. Insulin resistance and risk of chronic kidney disease in nondiabetic US adults. J Am Soc Nephrol. 2003. 14:469–477.32. Kurella M, Lo JC, Chertow GM. Metabolic syndrome and the risk for chronic kidney disease among nondiabetic adults. J Am Soc Nephrol. 2005. 16:2134–2140.33. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998. 15:539–553.34. Koçak H, Oner-Iyidoğan Y, Güdöl F, Koçak T, Esin D. The relation between serum MDA and cystatin C levels in chronic spinal cord injury patients. Clin Biochem. 2005. 38:1034–1037.35. Nishiyama K, Konishi A, Nishio C, Araki-Yoshida K, Hatanaka H, Kojima M, Ohmiya Y, Yamada M, Koshimizu H. Expression of cystatin C prevents oxidative stress-induced death in PC12 cells. Brain Res Bull. 2005. 67:94–99.36. Demircan N, Gurel A, Armutcu F, Unalacak M, Aktunc E, Atmaca H. The evaluation of serum cystatin C, malondialdehyde, and total antioxidant status in patients with metabolic syndrome. Med Sci Monit. 2008. 14:CR97–CR101.37. Alberti KG, Zimmet P, Shaw J. The metabolic syndrome: a new worldwide definition. Lancet. 2005. 366:1059–1062.38. Choi KM, Kim SM, Kim YE, Choi DS, Baik SH, Lee J. International Diabetes Federation: Prevalence and cardiovascular disease risk of the metabolic syndrome using National Cholesterol Education Program and International Diabetes Federation definitions in the Korean population. Metabolism. 2007. 56:552–558.39. Yoon YS, Lee ES, Park C, Lee S, Oh SW. The new definition of metabolic syndrome by the international diabetes federation is less likely to identify metabolically abnormal but non-obese individuals than the definition by the revised national cholesterol education program: the Korea NHANES study. Int J Obes (Lond). 2007. 31:528–534.40. Lee S, Park HS, Kim SM, Kwon HS, Kim DY, Kim DJ, Cho GJ, Han JH, Kim SR, Park CY, Oh SJ, Lee CB, Kim KS, Oh SW, Kim YS, Choi WH, Yoo HJ. Cut-off Points of Waist Circumference for Defining Abdominal Obesity in the Korean Population. Korean J Obes. 2006. 15:1–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Association of Obesity with Serum Cystatin C in Korean Adults
- Letter: The Association of Serum Cystatin C with Glycosylated Hemoglobin in Korean Adults (Diabetes Metab J 2016;40:62-9)
- Prevalence of Reduced Kidney Function by Estimated Glomerular Filtration Rate Using an Equation Based on Creatinine and Cystatin C in Metabolic Syndrome and Its Components in Korean Adults
- The relationship between metabolic syndrome components and the number of remaining teeth in Korean adults
- Response: The Association of Serum Cystatin C with Glycosylated Hemoglobin in Korean Adults (Diabetes Metab J 2016;40:62-9)