Korean J Urol.
2006 Aug;47(8):882-887. 10.4111/kju.2006.47.8.882.
Molecular MRI Images of the Transplanted Human Mesenchymal Stem Cells in the Liver, Kidney, Bladder and Penile Cavernosum of Rats
- Affiliations
-
- 1Department of Urology, Soonchunhyang University College of Medicine, Seoul, Korea.
- 2Stem Cell Therapy Center, Soonchunhyang University College of Medicine, Seoul, Korea. jhwon@hosp.sch.ac.kr
- 3Department of Radiology, Soonchunhyang University College of Medicine, Seoul, Korea.
- 4Department of Surgery, Soonchunhyang University College of Medicine, Seoul, Korea.
- 5Department of Internal Medicine, Soonchunhyang University College of Medicine, Seoul, Korea.
- 6Department of Obstetrics and Gynecology, Inha University College of Medicine, Incheon, Korea.
- KMID: 2294119
- DOI: http://doi.org/10.4111/kju.2006.47.8.882
Abstract
-
PURPOSE: Molecular magnetic resonance (MR) imaging techniques using superparamagnetic iron oxide nanocrystals (SPIO) have been developed for noninvasively monitoring stem cells. This study was performed to investigate if the presence of transplanted human mesenchymal stem cells in the liver, kidney, bladder and penile cavernosum can be evaluated noninvasively with using molecular MR imaging.
MATERIALS AND METHODS
SPIO (Feridex; AMI, Cambridge, USA) were transferred to the human mesenchymal stem cells (hMSCs) using GenePORTER. The labeling viability, efficiency and differentiation of the SPIO transferred hMSCs were examined with Tripan blue, Von Kossa, alkaline phosphatase, toluidine blue, oil red O and Prussian blue staining. The SPIO labelled hMSCs were transplanted to the liver, kidney, bladder and penile cavernosum of rats, and the MR images were examined in vitro or in vivo using 1.5 T MR.
RESULTS
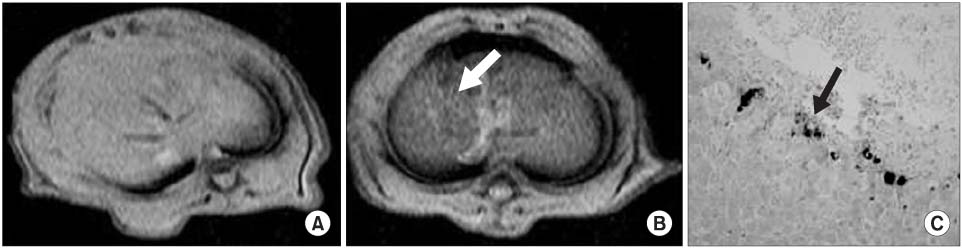
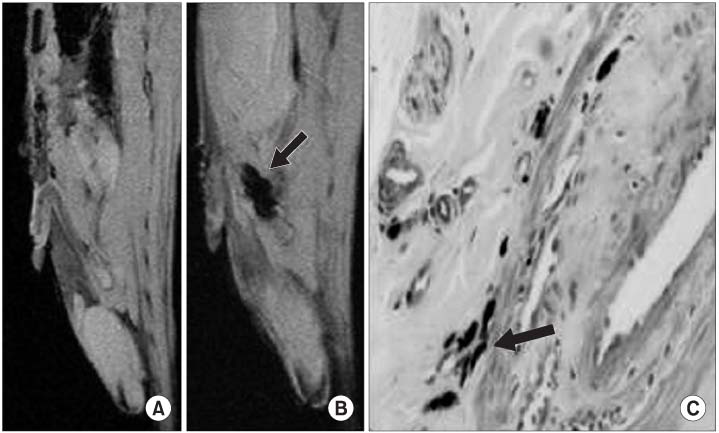
The viability and efficiency of the SPIO transferred hMSCs were good. Osteogenic, chondrogenic or adipogenic differentiation from the SPIO transferred hMSCs was observed. A decrease of the MR signal intensity of the SPIO transferred hMSCs with using GenePORTER was found in vitro. A decrease of the MR signal intensity was found at concentrations that were more than 1x10(5) hMSCs in vitro. The MR signal intensity at the areas of the SPIO transferred hMSCs decreased in the liver, kidney, bladder and penile cavernosum. The intracellular SPIOs were confirmed in the SPIO labelled hMSCs that were transplanted in the liver, kidney, bladder and penile cavernosum with Prussian blue staining.
CONCLUSIONS
The SPIO labelled hMSCs in the liver, kidney, bladder and penis can be evaluated noninvasively by using molecular MRI.
MeSH Terms
Figure
Reference
-
1. Ittrich H, Lange C, Dahnke H, Zander AR, Adam G, Nolte-Ernsting C. Labeling of mesenchymal stem cells with different superparamagnetic particles of iron oxide and detectability with MRI at 3T. Rofo. 2005. 177:1151–1163.2. Matuszewski L, Persigehl T, Wall A, Schwindt W, Tombach B, Fobker M, et al. Cell tagging with clinically approved iron oxides: feasibility and effect of lipofection, particle size, and surface coating on labeling efficiency. Radiology. 2005. 235:155–161.3. Unger EC. How can superparamagnetic iron oxides be used to monitor disease and treatment? Radiology. 2003. 229:615–616.4. Tang YL, Zhao Q, Zhang YC, Cheng L, Liu M, Shi J, et al. Autologous mesenchymal stem cell transplantation induce VEGF and neovascularization in ischemic myocardium. Regul Pept. 2004. 117:3–10.5. Nagaya N, Kangawa K, Itoh T, Iwase T, Murakami S, Miyahara Y, et al. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005. 112:1128–1135.6. Park SK, Won JH, Park SJ, Chung NG, Jeong DC, Kim CK, et al. The role of mesenchymal stem cells in hematopoietic stem cell transplantation. Korean J Med. 2003. 65:277–288.7. Arbab AS, Yocum GT, Wilson LB, Parwana A, Jordan EK, Kalish H, et al. Comparison of transfection agents in forming complexes with ferumoxides, cell labeling efficiency, and cellular viability. Mol Imaging. 2004. 3:24–32.8. Djurovic S, Iversen N, Jeansson S, Hoover F, Christensen G. Comparison of nonviral transfection and adeno-associated viral transduction on cardiomyocytes. Mol Biotechnol. 2004. 28:21–32.9. Kostura L, Kraitchman DL, Mackay AM, Pittenger MF, Bulte JW. Feridex labeling of mesenchymal stem cells inhibits chondrogenesis but not adipogenesis or osteogenesis. NMR Biomed. 2004. 17:513–517.10. Nishida M, Higuchi H, Kobayashi Y, Takagishi K. Hitological and biochemical changes of experimental meniscus tear in the dog knee. J Orthop Sci. 2005. 10:406–413.11. Yang M, Ma QJ, Dang GT, Ma K, Chen P, Zhou CY. In vitro and in vivo induction of bone formation based on ex vivo gene therapy using rat adipose-derived adult stem cells expressing BMP-7. Cytotherapy. 2005. 7:273–281.12. Kang X, Xie Y, Kniss DA. Adipose tissue model using three-dimensional cultivation of preadipocytes seeded onto fibrous polymer scaffolds. Tissue Eng. 2005. 11:458–468.13. Oweida AJ, Dunn EA, Foster PJ. Cellular imaging at 1.5T: detecting cells in neuroinflammation using active labeling with superparamagnetic iron oxide. Mol Imaging. 2004. 3:85–89.14. Fleige G, Nolte C, Synowitz M, Seeberger F, Kettenmann H, Zimmer C. Magnetic labeling of activated microglia in experimental gliomas. Neoplasia. 2001. 3:489–499.15. Bremer C, Allkemper T, Baerming J, Reimer P. RES-specific imaging of the liver and spleen with iron oxide particles designed for blood pool MR-angiography. J Magn Reson Imaging. 1999. 10:461–467.16. Lewin M, Carlesso N, Tung CH, Tanf XW, Cory D, Scadden DT, et al. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat Biotechnol. 2000. 18:410–414.17. Kraitchman DL, Heldman AW, Atalar E, Adamo LC, Martin BJ, Pittenger MF, et al. In vivo magnetic resonance image of mesenchymal stem cells in myocardial infarction. Circulation. 2003. 107:2290–2293.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Monitoring Transplanted Human Mesenchymal Stem Cells in the Penile Cavernosal Tissues of Streptozotocin-induced Diabetic Rats Using Molecular Magnetic Resonance Imaging
- Histologic Monitoring of the Transplanted Superparamagnetic Iron Oxide Labelled Human Mesenchymal Stem Cells in the Rat Bladder
- Monitoring Transplanted Human Mesenchymal Stem Cells in the Rabbit Bladder Using Molecular MRI
- Use of nanoparticles to monitor human mesenchymal stem cells transplanted into penile cavernosum of rats with erectile dysfunction
- Periosteum-Derived Mesenchymal Stem Cells and Scaffolds Transplanted into Long-Bone Defects of Rabbit