Korean J Urol.
2015 Apr;56(4):280-287. 10.4111/kju.2015.56.4.280.
Use of nanoparticles to monitor human mesenchymal stem cells transplanted into penile cavernosum of rats with erectile dysfunction
- Affiliations
-
- 1Department of Urology, Soonchunhyang University Seoul Hospital, Soonchunhyang University College of Medicine, Seoul, Korea. yssong@schmc.ac.kr
- 2Medical Research Institute, Chung-Ang University College of Medicine, Seoul, Korea.
- 3Department of Surgery, Hanyang University School of Medicine, Seoul, Korea.
- 4Department of Radiology, Seoul National University College of Medicine, Seoul, Korea.
- 5Department of Oncology and Hematology, Soonchunhyang University Seoul Hospital, Soonchunhyang University College of Medicine, Seoul, Korea.
- KMID: 2155303
- DOI: http://doi.org/10.4111/kju.2015.56.4.280
Abstract
- PURPOSE
This study was performed to examine the treatment of erectile dysfunction by use of superparamagnetic iron oxide nanoparticles-labeled human mesenchymal stem cells (SPION-MSCs) transplanted into the cavernous nerve injured cavernosa of rats as monitored by molecular magnetic resonance imaging (MRI).
MATERIALS AND METHODS
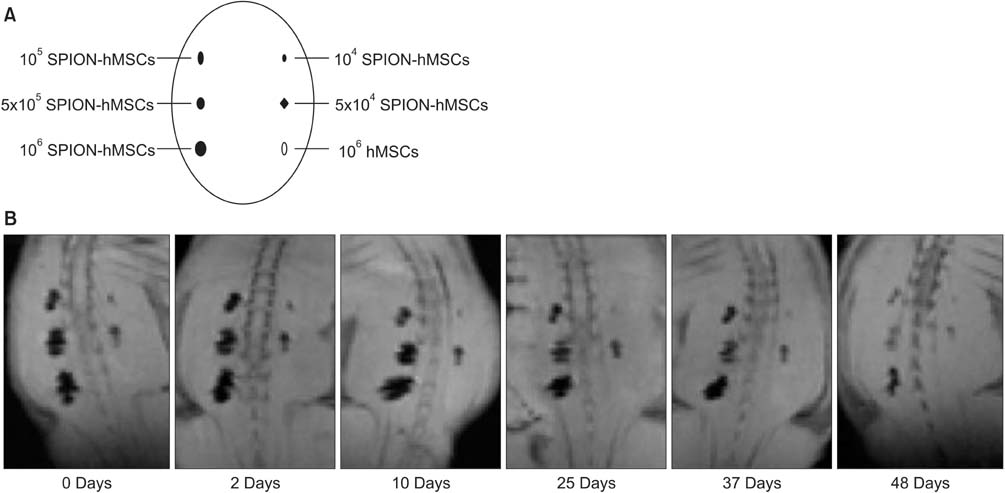
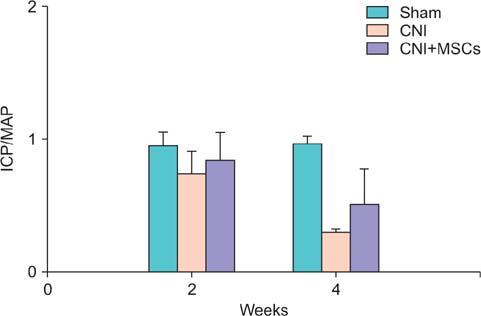
Eight-week-old male Sprague-Dawley rats were divided into three groups of 10 rats each: group 1, sham operation; group 2, cavernous nerve injury; group 3, SPION-MSC treatment after cavernous nerve injury. Immediately after the cavernous nerve injury in group 3, SPION-MSCs were injected into the cavernous nerve injured cavernosa. Serial T2-weighted MRI was done immediately after injection and at 2 and 4 weeks. Erectile response was assessed by cavernous nerve stimulation at 2 and 4 weeks.
RESULTS
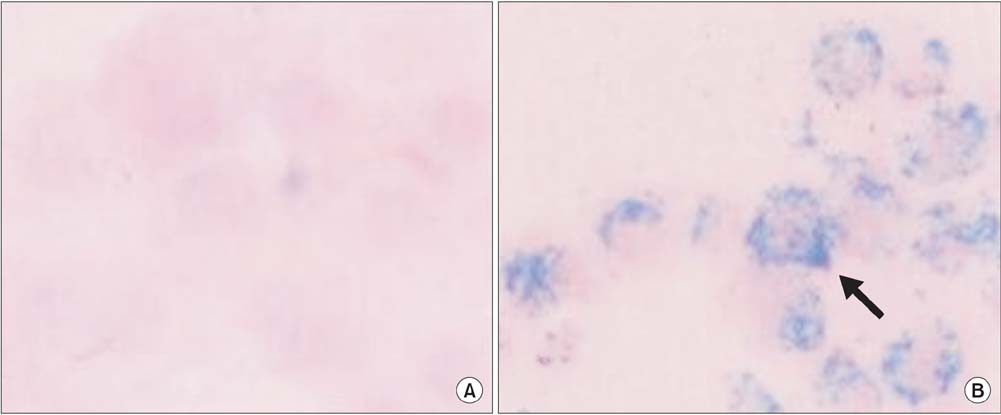
Prussian blue staining of SPION-MSCs revealed abundant uptake of SPION in the cytoplasm. After injection of 1x10(6) SPION-MSCs into the cavernosa of rats, T2-weighted MRI showed a clear hypointense signal induced by the injection. The presence of SPION in the corpora cavernosa was confirmed with Prussian blue staining. At 2 and 4 weeks, rats with cavernous nerve injury had significantly lower erectile function than did rats without cavernous nerve injury (p<0.05). The group transplanted with SPION-MSCs showed higher erectile function than did the group without SPION-MSCs (p<0.05). The presence of SPION-MSCs for up to 4 weeks was confirmed by MRI imaging and Prussian blue staining in the corpus cavernosa.
CONCLUSIONS
Transplanted SPION-MSCs existed for up to 4 weeks in the cavernous nerve injured cavernosa of rats. Erectile dysfunction recovered and could be monitored by MRI.
Keyword
MeSH Terms
-
Animals
Contrast Media/pharmacology
Dextrans/*pharmacology
Disease Models, Animal
Drug Delivery Systems/methods
*Erectile Dysfunction/diagnosis/etiology/therapy
Magnetic Resonance Imaging/methods
*Magnetite Nanoparticles
Male
Mesenchymal Stem Cell Transplantation/*methods
Monitoring, Physiologic/methods
Penis/*innervation
*Peripheral Nerve Injuries/complications/diagnosis/physiopathology/therapy
Rats
Suspensions
Treatment Outcome
Contrast Media
Dextrans
Magnetite Nanoparticles
Suspensions
Figure
Reference
-
1. Quinlan DM, Epstein JI, Carter BS, Walsh PC. Sexual function following radical prostatectomy: influence of preservation of neurovascular bundles. J Urol. 1991; 145:998–1002.2. Hatzimouratidis K, Burnett AL, Hatzichristou D, McCullough AR, Montorsi F, Mulhall JP. Phosphodiesterase type 5 inhibitors in postprostatectomy erectile dysfunction: a critical analysis of the basic science rationale and clinical application. Eur Urol. 2009; 55:334–347.3. Montorsi F, Brock G, Lee J, Shapiro J, Van Poppel H, Graefen M, et al. Effect of nightly versus on-demand vardenafil on recovery of erectile function in men following bilateral nerve-sparing radical prostatectomy. Eur Urol. 2008; 54:924–931.4. Fall PA, Izikki M, Tu L, Swieb S, Giuliano F, Bernabe J, et al. Apoptosis and effects of intracavernous bone marrow cell injection in a rat model of postprostatectomy erectile dysfunction. Eur Urol. 2009; 56:716–725.5. Kendirci M, Trost L, Bakondi B, Whitney MJ, Hellstrom WJ, Spees JL. Transplantation of nonhematopoietic adult bone marrow stem/progenitor cells isolated by p75 nerve growth factor receptor into the penis rescues erectile function in a rat model of cavernous nerve injury. J Urol. 2010; 184:1560–1566.6. Ittrich H, Lange C, Dahnke H, Zander AR, Adam G, Nolte-Ernsting C. Labeling of mesenchymal stem cells with different superparamagnetic particles of iron oxide and detectability with MRI at 3T. Rofo. 2005; 177:1151–1163.7. Matuszewski L, Persigehl T, Wall A, Schwindt W, Tombach B, Fobker M, et al. Cell tagging with clinically approved iron oxides: feasibility and effect of lipofection, particle size, and surface coating on labeling efficiency. Radiology. 2005; 235:155–161.8. Bulte JW, Duncan ID, Frank JA. In vivo magnetic resonance tracking of magnetically labeled cells after transplantation. J Cereb Blood Flow Metab. 2002; 22:899–907.9. Song YS, Ku JH, Song ES, Kim JH, Jeon JS, Lee KH, et al. Magnetic resonance evaluation of human mesenchymal stem cells in corpus cavernosa of rats and rabbits. Asian J Androl. 2007; 9:361–367.10. Song YS, Ku JH. Monitoring transplanted human mesenchymal stem cells in rat and rabbit bladders using molecular magnetic resonance imaging. Neurourol Urodyn. 2007; 26:584–593.11. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284:143–147.12. Lee HJ, Won JH, Doo SH, Kim JH, Song KY, Lee SJ, et al. Inhibition of collagen deposit in obstructed rat bladder outlet by transplantation of superparamagnetic iron oxide-labeled human mesenchymal stem cells as monitored by molecular magnetic resonance imaging (MRI). Cell Transplant. 2012; 21:959–970.13. Nagai A, Kim WK, Lee HJ, Jeong HS, Kim KS, Hong SH, et al. Multilineage potential of stable human mesenchymal stem cell line derived from fetal marrow. PLoS One. 2007; 2:e1272.14. Song YS, Lee HJ, Park IH, Kim WK, Ku JH, Kim SU. Potential differentiation of human mesenchymal stem cell transplanted in rat corpus cavernosum toward endothelial or smooth muscle cells. Int J Impot Res. 2007; 19:378–385.15. Iacono F, Giannella R, Somma P, Manno G, Fusco F, Mirone V. Histological alterations in cavernous tissue after radical prostatectomy. J Urol. 2005; 173:1673–1676.16. Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A. 2000; 97:3422–3427.17. Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001; 410:701–705.18. Woo LL, Tanaka ST, Anumanthan G, Pope JC 4th, Thomas JC, Adams MC, et al. Mesenchymal stem cell recruitment and improved bladder function after bladder outlet obstruction: preliminary data. J Urol. 2011; 185:1132–1138.19. Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001; 169:12–20.20. Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP. Stem cell transplantation: the lung barrier. Transplant Proc. 2007; 39:573–576.21. Liechty KW, MacKenzie TC, Shaaban AF, Radu A, Moseley AM, Deans R, et al. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000; 6:1282–1286.22. Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004; 95:9–20.23. Verfaillie CM. Adult stem cells: assessing the case for pluripotency. Trends Cell Biol. 2002; 12:502–508.24. Sharma AD, Cantz T, Manns MP, Ott M. The role of stem cells in physiology, pathophysiology, and therapy of the liver. Stem Cell Rev. 2006; 2:51–58.25. Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004; 109:1543–1549.26. Silva GV, Litovsky S, Assad JA, Sousa AL, Martin BJ, Vela D, et al. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005; 111:150–156.27. Bulte JW, Douglas T, Witwer B, Zhang SC, Strable E, Lewis BK, et al. Magnetodendrimers allow endosomal magnetic labeling and in vivo tracking of stem cells. Nat Biotechnol. 2001; 19:1141–1147.28. Kraitchman DL, Heldman AW, Atalar E, Amado LC, Martin BJ, Pittenger MF, et al. In vivo magnetic resonance imaging of mesenchymal stem cells in myocardial infarction. Circulation. 2003; 107:2290–2293.29. Kostura L, Kraitchman DL, Mackay AM, Pittenger MF, Bulte JW. Feridex labeling of mesenchymal stem cells inhibits chondrogenesis but not adipogenesis or osteogenesis. NMR Biomed. 2004; 17:513–517.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Monitoring Transplanted Human Mesenchymal Stem Cells in the Penile Cavernosal Tissues of Streptozotocin-induced Diabetic Rats Using Molecular Magnetic Resonance Imaging
- Stem Cell Therapy for Erectile Dysfunction
- Molecular MRI Images of the Transplanted Human Mesenchymal Stem Cells in the Liver, Kidney, Bladder and Penile Cavernosum of Rats
- The Expression of eNOS and ET-1 in Corpus Cavernosum in Male Rat with Partial Bladder Outlet Obstruction
- Adipose Derived Mesenchymal Stem Cells-Derived Mitochondria Transplantation Ameliorated Erectile Dysfunction Induced by Cavernous Nerve Injury