J Clin Neurol.
2008 Dec;4(4):148-152. 10.3988/jcn.2008.4.4.148.
Cilostazol Reduces PAC-1 Expression on Platelets in Ischemic Stroke
- Affiliations
-
- 1Department of Neurology, College of Medicine, Dong-A University, Busan, Korea. nrcjk@unitel.co.kr
- 2Department of Radiology, College of Medicine, Dong-A University, Busan, Korea.
- KMID: 2287667
- DOI: http://doi.org/10.3988/jcn.2008.4.4.148
Abstract
- BACKGROUND AND PURPOSE
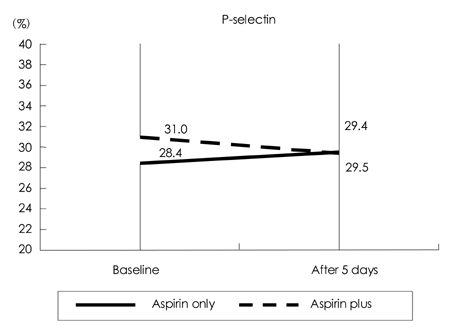
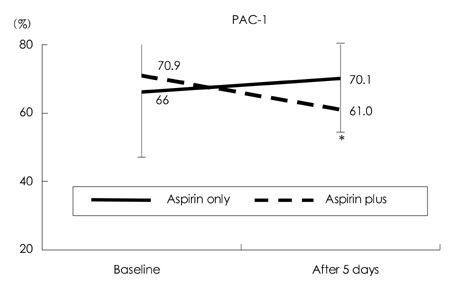
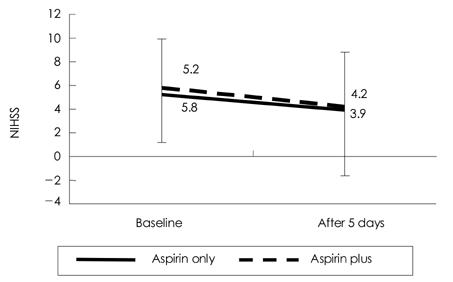
Cilostazol, a phosphodiesterase III inhibitor, is known to be a useful antiplatelet agent that inhibits the progression of atherosclerosis in ischemic stroke. This study investigated the effects of combining cilostazol with aspirin on the expressions of P-selectin and PAC-1 on activated platelets in acute ischemic stroke. METHODS: We analyzed 70 patients with acute ischemic stroke (<72 hrs of an ischemic event). The daily intake was 100 mg of aspirin in 37 patients and 100 mg of aspirin plus 200 mg of cilostazol in 33 patients. The expressions of P-selectin and PAC-1 on activated platelets were measured on the day of admission and 5 days later. We also evaluated the clinical progression using the National Institutes of Health Stroke Scale (NIHSS) at the same times. RESULTS: After 5 days the extent of PAC-1 expression on activated platelets was significantly lower for combined aspirin and cilostazol treatment (61.0+/-19.3%, p=0.008; mean+/-standard deviation) than the baseline level (70.9+/-12.9%), but did not differ between aspirin alone (66.0 +/-19.0%) and baseline (70.1+/-15.7%). The expression of P-selectin did not differ between combined aspirin and cilostazol treatment and baseline. The clinical progression did not differ between the two groups, as indicated by the absence of significant changes on the NIHSS in the acute period. CONCLUSIONS:This study found that the combined regimen of aspirin and cilostazol exerts the beneficial effect of reducing PAC-1 activity on activated platelets in acute ischemic stroke. However, the clinical outcome of this regimen was no better than that of the aspirin-only regimen. Therefore, further detailed studies of the possible clinical benefits of cilostazol in acute ischemic stroke are needed.
Keyword
MeSH Terms
Figure
Reference
-
1. Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999. 340:115–126.2. Chen ZM, Sandercock P, Pan HC, Counsell C, Collins R, Liu LS, et al. Indications for early aspirin use in acute ischemic stroke: a combined analysis of 40,000 randomized patients from the chinese acute stroke trial and the international stroke trial. On behalf of the CAST and IST collaborative groups. Stroke. 2000. 31:1240–1249.
Article3. Pereira AC, Brown MM. Aspirin or heparin in acute stroke. Br Med Bull. 2000. 56:413–421.
Article4. Markus HS. Current treatments in neurology: stroke. J Neurol. 2005. 252:260–267.
Article5. Norris JW. Antiplatelet agents in secondary prevention of stroke: a perspective. Stroke. 2005. 36:2034–2036.
Article6. Kwon SU, Cho YJ, Koo JS, Bae HJ, Lee YS, Hong KS, et al. Cilostazol Prevents the progression of the symptomatic intracranial arterial stenosis: the multicenter double-blind placebo-controlled trial of cilostazol in symptomatic intracranial arterial stenosis. Stroke. 2005. 36:782–786.
Article7. Goldstein LB, Jones MR, Matchar DB, Edwards LJ, Hoff J, Chilukuri V, et al. Improving the reliability of stroke subgroup classification using the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) criteria. Stroke. 2001. 32:1091–1098.
Article8. Leira EC, Chang KC, Davis PH, Clarke WR, Woolson RF, Hansen MD, et al. Can we predict early recurrence in acute stroke? Cerebrovasc Dis. 2004. 18:139–144.
Article9. Lovett JK, Coull AJ, Rothwell PM. Early risk of recurrence by subtype of ischemic stroke in population-based incidence studies. Neurology. 2004. 62:569–573.
Article10. Cha JK, Jeong MH, Lee KM, Bae HR, Lim YJ, Park KW, et al. Changes in platelet P-selectin and in plasma C-reactive protein in acute atherosclerotic ischemic stroke treated with a loading dose of clopidogrel. J Thromb Thrombolysis. 2002. 14:145–150.11. Diener HC, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebocontrolled trial. Lancet. 2004. 364:331–337.
Article12. ESPRIT Study Group. Halkes PH, van Gijn J, Kappelle LJ, Koudstaal PJ, Algra A. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet. 2006. 367:1665–1673.
Article13. Zambahari R, Kwok OH, Javier S, Mak KH, Piyamitr S, Tri Ho HQ, et al. Clinical use of clopidogrel in acute coronary syndrome. Int J Clin Pract. 2007. 61:473–481.
Article14. Bhatt DL, Topol EJ. Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance Executive Committee. Clopidogrel added to aspirin versus aspirin alone in secondary prevention and high-risk primary prevention: rationale and design of the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial. Am Heart J. 2004. 148:263–268.
Article15. Yamasaki Y, Kim YS, Kawamori R. Rationale and protocol of a trial for prevention of diabetic atherosclerosis by using antiplatelet drugs: study of Diabetic Atherosclerosis Prevention by Cilostazol (DAPC study). Cardiovasc Diabetol. 2006. 5:16.16. Dalainas I. Cilostazol in the management of vascular disease. Int Angiol. 2007. 26:1–7.17. Steinhubl SR, Moliterno DJ. The role of the platelet in the pathogenesis of atherothrombosis. Am J Cardiovasc Drugs. 2005. 5:399–408.
Article18. Hagberg IA, Lyberg T. Blood platelet activation evaluated by flow cytometry: optimised methods for clinical studies. Platelets. 2000. 11:137–150.
Article19. Patrignani P, Filabozzi P, Patrono C. Selective cumulative inhibition of platelet thromboxane production by low-dose aspirin in healthy subjects. J Clin Invest. 1982. 69:1366–1372.
Article20. Pulcinelli FM, Riondino S, Celestini A, Pignatelli P, Trifiró E, Di Renzo L, et al. Persistent production of platelet thromboxane A2 in patients chronically treated with aspirin. J Thromb Haemost. 2005. 3:2784–2789.
Article21. Wong S, Appleberg M, Ward CM, Lewis DR. Aspirin resistance in cardiovascular disease: a review. Eur J Vasc Endovasc Surg. 2004. 27:456–465.
Article22. Mason PJ, Jacobs AK, Freedman JE. Aspirin resistance and atherothrombotic disease. J Am Coll Cardiol. 2005. 46:986–993.
Article23. Nomura S, Inami N, Iwasaka T, Liu Y. Platelet activation markers, microparticles and soluble adhesion molecules are elevated in patients with arteriosclerosis obliterans: therapeutic effects by cilostazol and potentiation by dipyridamole. Platelets. 2004. 15:167–172.
Article24. Serebruany VL, Malinin AI, Ziai W, Pokov AN, Bhatt DL, Alberts MJ, et al. Effects of clopidogrel and aspirin in combination versus aspirin alone on platelet activation and major receptor expression in patients after recent ischemic stroke: for the Plavix Use for Treatment of Stroke (PLUTO-Stroke) trial. Stroke. 2005. 36:2289–2292.
Article25. May AE, Langer H, Seizer P, Bigalke B, Lindemann S, Gawaz M. Platelet-leukocyte interactions in inflammation and atherothrombosis. Semin Thromb Hemost. 2007. 33:123–127.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Change of PAC-1 and P-selectin Expression after Initiation of Antiplatelets in Patients with Acute Ischemic Stroke
- Surface Expression of P-selectin on Platelets Is Related with Clinical Worsening in Acute Ischemic Stroke
- The Serial Change of Platelet Activation for 90 Days in Patients with Atherosclerotic Ischemic Stroke
- Aspirin for the Prevention of Cardiovascular Events
- Antiplatelet Therapy for Secondary Stroke Prevention: 2012 Focused Update of Korean Clinical Practice Guidelines for Stroke