J Breast Cancer.
2012 Dec;15(4):420-426. 10.4048/jbc.2012.15.4.420.
Multiple Margin Positivity of Frozen Section Is an Independent Risk Factor for Local Recurrence in Breast-Conserving Surgery
- Affiliations
-
- 1Department of Surgery, Pusan National University Hospital, Busan, Korea. bytae@pusan.ac.kr
- KMID: 2286439
- DOI: http://doi.org/10.4048/jbc.2012.15.4.420
Abstract
- PURPOSE
Breast-conserving surgery (BCS) with radiotherapy has become a standard treatment for early stage breast cancer, since the installation of NSABP B-06. One of the serious problems in BCS is that of local recurrence. There are many risk factors for local recurrence, such as large tumor size, multiple tumors, axillary lymph node involvement, young age, high nuclear grade, and so on. The aim of this study is to identify patients with a higher risk of local recurrence of breast cancer.
METHODS
Between January 2002 and December 2006, 447 patients with breast cancer, and who had undergone BCS with immediate breast reconstruction, were enrolled in the study. The follow-up period was 5 years from the time of operation and we analyzed local recurrence, disease-free survival (DFS), and overall survival (OS). The analysis included various clinicopathological factors such as age, chemotherapy, radiotherapy, hormone therapy, pathologic characteristics, and margin status. Statistical analysis was performed with log-rank test and Kaplan-Meier method. The p-value <0.05 was considered statistically significant.
RESULTS
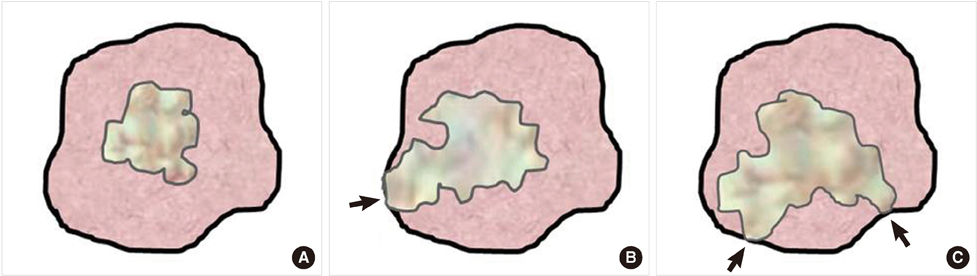
The mean follow-up period was 88 months and local recurrence of breast cancer occurred only in 16 cases (3.6%). The actual 5-year DFS, and OS rates were 90.6% and 93.3%, respectively. For the local recurrence of breast cancer, positive margin status, multiple margin positivity, conversed margin cases, T/N stages showed statistical significance in univariate analysis. However, only multiple margin positivity was identified as an independent risk factor for local recurrence in multivariate analysis.
CONCLUSION
When the multiple margin positivity is diagnosed on intraoperative frozen biopsy, surgeons should consider a much wider excision of the breast and a more aggressive management.
MeSH Terms
Figure
Reference
-
1. Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002. 347:1233–1241.
Article2. Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002. 347:1227–1232.
Article3. Jacobson JA, Danforth DN, Cowan KH, d'Angelo T, Steinberg SM, Pierce L, et al. Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer. N Engl J Med. 1995. 332:907–911.
Article4. van Dongen JA, Voogd AC, Fentiman IS, Legrand C, Sylvester RJ, Tong D, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000. 92:1143–1150.
Article5. Mirza NQ, Vlastos G, Meric F, Buchholz TA, Esnaola N, Singletary SE, et al. Predictors of locoregional recurrence among patients with early-stage breast cancer treated with breast-conserving therapy. Ann Surg Oncol. 2002. 9:256–265.
Article6. van den Broek N, van der Sangen MJ, van de Poll-Franse LV, van Beek MW, Nieuwenhuijzen GA, Voogd AC. Margin status and the risk of local recurrence after breast-conserving treatment of lobular breast cancer. Breast Cancer Res Treat. 2007. 105:63–68.
Article7. Ikeda T, Akiyama F, Hiraoka M, Inaji H, Ohuchi N, Takatsuka Y, et al. Surgical margin status as a cause of local failure after breast conserving therapy. Breast Cancer. 1999. 6:93–97.
Article8. Luini A, Rososchansky J, Gatti G, Zurrida S, Caldarella P, Viale G, et al. The surgical margin status after breast-conserving surgery: discussion of an open issue. Breast Cancer Res Treat. 2009. 113:397–402.
Article9. Fredriksson I, Liljegren G, Palm-Sjövall M, Arnesson LG, Emdin SO, Fornander T, et al. Risk factors for local recurrence after breast-conserving surgery. Br J Surg. 2003. 90:1093–1102.
Article10. Kurtz JM, Amalric R, Brandone H, Ayme Y, Spitalier JM. Contralateral breast cancer and other second malignancies in patients treated by breast-conserving therapy with radiation. Int J Radiat Oncol Biol Phys. 1988. 15:277–284.
Article11. Katz A, Strom EA, Buchholz TA, Thames HD, Smith CD, Jhingran A, et al. Locoregional recurrence patterns after mastectomy and doxorubicin-based chemotherapy: implications for postoperative irradiation. J Clin Oncol. 2000. 18:2817–2827.
Article12. Kurtz JM, Jacquemier J, Amalric R, Brandone H, Ayme Y, Hans D, et al. Why are local recurrences after breast-conserving therapy more frequent in younger patients? J Clin Oncol. 1990. 8:591–598.
Article13. Müller A, von Fournier D, Kaufmann M, Otto HF, Abel U. Whole breast irradiation and boost irradiation in breast-conserving therapy based on morphologic findings. Breast Dis. 1989. 2:121–130.14. Cowen D, Jacquemier J, Houvenaeghel G, Viens P, Puig B, Bardou VJ, et al. Local and distant recurrence after conservative management of "very low-risk" breast cancer are dependent events: a 10-year follow-up. Int J Radiat Oncol Biol Phys. 1998. 41:801–807.
Article15. Leopold KA, Recht A, Schnitt SJ, Connolly JL, Rose MA, Silver B, et al. Results of conservative surgery and radiation therapy for multiple synchronous cancers of one breast. Int J Radiat Oncol Biol Phys. 1989. 16:11–16.
Article16. Kurtz JM, Jacquemier J, Amalric R, Brandone H, Ayme Y, Hans D, et al. Breast-conserving therapy for macroscopically multiple cancers. Ann Surg. 1990. 212:38–44.
Article17. Calle R, Vilcoq JR, Zafrani B, Vielh P, Fourquet A. Local control and survival of breast cancer treated by limited surgery followed by irradiation. Int J Radiat Oncol Biol Phys. 1986. 12:873–878.
Article18. Stotter AT, McNeese MD, Ames FC, Oswald MJ, Ellerbroek NA. Predicting the rate and extent of locoregional failure after breast conservation therapy for early breast cancer. Cancer. 1989. 64:2217–2225.
Article19. Clark RM, McCulloch PB, Levine MN, Lipa M, Wilkinson RH, Mahoney LJ, et al. Randomized clinical trial to assess the effectiveness of breast irradiation following lumpectomy and axillary dissection for node-negative breast cancer. J Natl Cancer Inst. 1992. 84:683–689.
Article20. Straus K, Lichter A, Lippman M, Danforth D, Swain S, Cowan K, et al. Results of the National Cancer Institute early breast cancer trial. J Natl Cancer Inst Monogr. 1992. (11):27–32.21. Veronesi U, Banfi A, Salvadori B, Luini A, Saccozzi R, Zucali R, et al. Breast conservation is the treatment of choice in small breast cancer: long-term results of a randomized trial. Eur J Cancer. 1990. 26:668–670.
Article22. Vicini FA, Kestin LL, Goldstein NS, Baglan KL, Pettinga JE, Martinez AA. Relationship between excision volume, margin status, and tumor size with the development of local recurrence in patients with ductal carcinoma-in-situ treated with breast-conserving therapy. J Surg Oncol. 2001. 76:245–254.
Article23. Taghian A, Mohiuddin M, Jagsi R, Goldberg S, Ceilley E, Powell S. Current perceptions regarding surgical margin status after breast-conserving therapy: results of a survey. Ann Surg. 2005. 241:629–639.
Article24. Houssami N, Macaskill P, Marinovich ML, Dixon JM, Irwig L, Brennan ME, et al. Meta-analysis of the impact of surgical margins on local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy. Eur J Cancer. 2010. 46:3219–3232.
Article25. Fukamachi K, Ishida T, Usami S, Takeda M, Watanabe M, Sasano H, et al. Total-circumference intraoperative frozen section analysis reduces margin-positive rate in breast-conservation surgery. Jpn J Clin Oncol. 2010. 40:513–520.
Article26. Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer. 1989. 63:181–187.
Article27. Swenson KK, Decher L, Haselow R, Farrell JB, Sperduto PW. Prognostic factors after conservative surgery and radiation therapy for early stage breast cancer. Am J Clin Oncol. 1998. 21:111–116.
Article28. Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol. 1995. 48:1503–1510.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Atypical Hyperplasia at the Margin of Frozen Sections from Breast-Conserving Surgery
- Intraoperative Specimen Mammography for Margin Assessment in Breast-Conserving Surgery
- Comparison of Outcomes of Standard and Oncoplastic Breast-Conserving Surgery
- Breast-Conserving Surgery With or Without Radiation Therapy for Early Breast Cancer
- The Usefulness of Intraoperative Circumferential Frozen-Section Analysis of Lumpectomy Margins in Breast-Conserving Surgery