Korean J Physiol Pharmacol.
2016 May;20(3):229-236. 10.4196/kjpp.2016.20.3.229.
Resveratrol attenuates 4-hydroxy-2-hexenal-induced oxidative stress in mouse cortical collecting duct cells
- Affiliations
-
- 1Department of Internal Medicine, Chonnam National University Medical School, Gwangju 61469, Korea. skimw@chonnam.ac.kr
- 2Department of Physiology, Chonnam National University Medical School, Gwangju 61469, Korea.
- KMID: 2285594
- DOI: http://doi.org/10.4196/kjpp.2016.20.3.229
Abstract
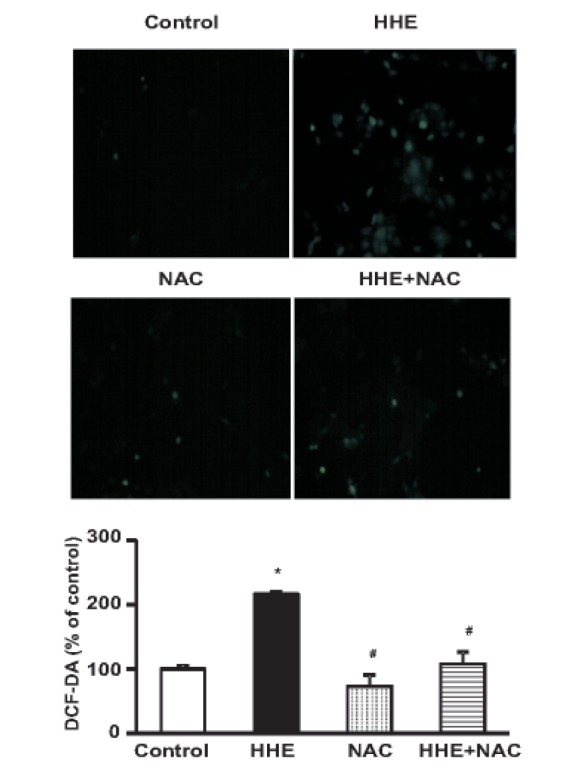
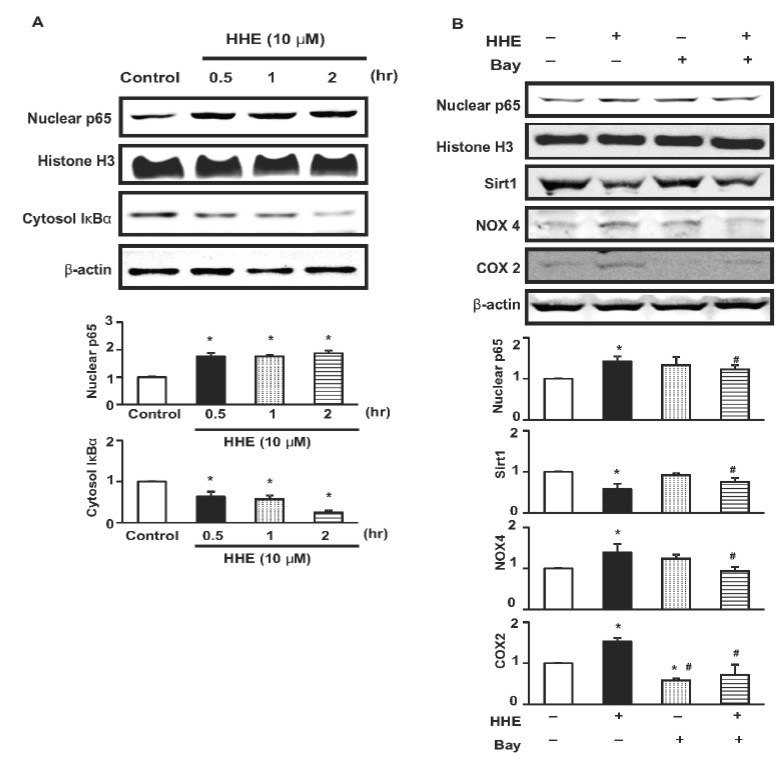
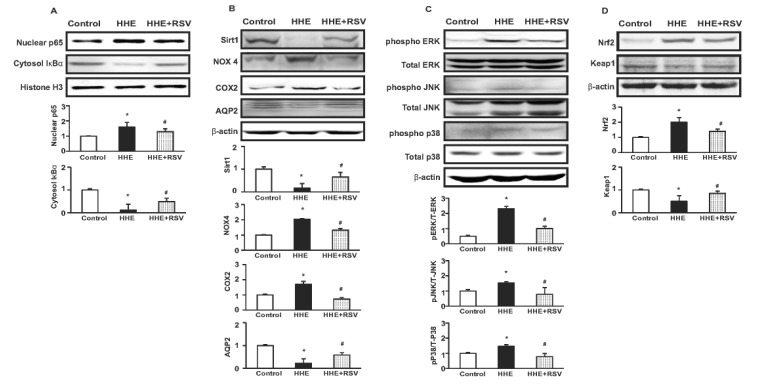
- Resveratrol (RSV) may provide numerous protective eff ects against chronic inflammatory diseases. Due to local hypoxia and hypertonicity, the renal medulla is subject to extreme oxidative stress, and aldehyde products formed during lipid peroxidation, such as 4-hydroxy-2-hexenal (HHE), might be responsible for tubular injury. This study aimed at investigating the eff ects of RSV on renal and its signaling mechanisms. While HHE treatment resulted in decreased expression of Sirt1, AQP2, and nuclear factor erythroid 2-related factor 2 (Nrf2), mouse cortical collecting duct cells (M1) cells treated with HHE exhibited increased activation of p38 MAPK, extracellular signal regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and increased expression of NOX4, p47(phox), Kelch ECH associating protein 1 (Keap1) and COX2. HHE treatment also induced NF-κB activation by promoting IκB-α degradation. Meanwhile, the observed increases in nuclear NF-κB, NOX4, p47(phox), and COX2 expression were attenuated by treatment with Bay 117082, N-acetyl-l-cysteine (NAC), or RSV. Our findings indicate that RSV inhibits the expression of inflammatory proteins and the production of reactive oxygen species in M1 cells by inhibiting NF-κB activation.
MeSH Terms
-
Acetylcysteine
Animals
Anoxia
Bays
JNK Mitogen-Activated Protein Kinases
Lipid Peroxidation
Mice*
Oxidative Stress*
p38 Mitogen-Activated Protein Kinases
Phosphotransferases
Reactive Oxygen Species
Sirtuin 1
Acetylcysteine
JNK Mitogen-Activated Protein Kinases
Phosphotransferases
Reactive Oxygen Species
Sirtuin 1
p38 Mitogen-Activated Protein Kinases
Figure
Reference
-
1. Parola M, Bellomo G, Robino G, Barrera G, Dianzani MU. 4-Hydroxynonenal as a biological signal: molecular basis and pathophysiological implications. Antioxid Redox Signal. 1999; 1:255–284. PMID: 11229439.
Article2. Domingues RM, Domingues P, Melo T, Pérez-Sala D, Reis A, Spickett CM. Lipoxidation adducts with peptides and proteins: deleterious modifications or signaling mechanisms? J Proteomics. 2013; 92:110–131. PMID: 23770299.
Article3. Bae EH, Cho S, Joo SY, Ma SK, Kim SH, Lee J, Kim SW. 4-Hydroxy-2-hexenal-induced apoptosis in human renal proximal tubular epithelial cells. Nephrol Dial Transplant. 2011; 26:3866–3873. PMID: 21771752.
Article4. Afshar G, Murnane JP. Characterization of a human gene with sequence homology to Saccharomyces cerevisiae SIR2. Gene. 1999; 234:161–168. PMID: 10393250.
Article5. Shi T, Wang F, Stieren E, Tong Q. SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J Biol Chem. 2005; 280:13560–13567. PMID: 15653680.
Article6. Guarente L, Picard F. Calorie restriction--the SIR2 connection. Cell. 2005; 120:473–482. PMID: 15734680.7. Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004; 23:2369–2380. PMID: 15152190.8. Harikumar KB, Aggarwal BB. Resveratrol: a multitargeted agent for age-associated chronic diseases. Cell Cycle. 2008; 7:1020–1035. PMID: 18414053.
Article9. Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006; 127:1109–1122. PMID: 17112576.10. Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007; 450:712–716. PMID: 18046409.
Article11. Trotter KW, Archer TK. Reconstitution of glucocorticoid receptor-dependent transcription in vivo. Mol Cell Biol. 2004; 24:3347–3358. PMID: 15060156.
Article12. Rosenau C, Emery D, Kaboord B, Qoronfleh MW. Development of a high-throughput plate-based chemiluminescent transcription factor assay. J Biomol Screen. 2004; 9:334–342. PMID: 15191650.
Article13. Temkin V, Karin M. From death receptor to reactive oxygen species and c-Jun N-terminal protein kinase: the receptor-interacting protein 1 odyssey. Immunol Rev. 2007; 220:8–21. PMID: 17979836.
Article14. Nakano H, Nakajima A, Sakon-Komazawa S, Piao JH, Xue X, Okumura K. Reactive oxygen species mediate crosstalk between NF-kappaB and JNK. Cell Death Differ. 2006; 13:730–737. PMID: 16341124.15. Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001; 410:37–40. PMID: 11242034.
Article16. Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995; 270:1326–1331. PMID: 7481820.
Article17. Kansanen E, Jyrkkänen HK, Levonen AL. Activation of stress signaling pathways by electrophilic oxidized and nitrated lipids. Free Radic Biol Med. 2012; 52:973–982. PMID: 22198184.
Article18. Kansanen E, Kuosmanen SM, Leinonen H, Levonen AL. The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox Biol. 2013; 1:45–49. PMID: 24024136.
Article19. Maheshwari A, Misro MM, Aggarwal A, Sharma RK, Nandan D. N-acetyl-L-cysteine counteracts oxidative stress and prevents H2O2 induced germ cell apoptosis through down-regulation of caspase-9 and JNK/c-Jun. Mol Reprod Dev. 2011; 78:69–79. PMID: 21254278.20. Karin M. Mitogen activated protein kinases as targets for development of novel anti-inflammatory drugs. Ann Rheum Dis. 2004; 63(Suppl 2):ii62–ii64. PMID: 15479874.
Article21. Hatada EN, Krappmann D, Scheidereit C. NF-kappaB and the innate immune response. Curr Opin Immunol. 2000; 12:52–58. PMID: 10679399.22. Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, Salminen A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal. 2013; 25:1939–1948. PMID: 23770291.
Article23. Salminen A, Kauppinen A, Suuronen T, Kaarniranta K. SIRT1 longevity factor suppresses NF-kappaB-driven immune responses: regulation of aging via NF-kappaB acetylation? Bioessays. 2008; 30:939–942. PMID: 18800364.24. Yang H, Zhang W, Pan H, Feldser HG, Lainez E, Miller C, Leung S, Zhong Z, Zhao H, Sweitzer S, Considine T, Riera T, Suri V, White B, Ellis JL, Vlasuk GP, Loh C. SIRT1 activators suppress inflammatory responses through promotion of p65 deacetylation and inhibition of NF-κB activity. PLoS One. 2012; 7:e46364. PMID: 23029496.
Article25. Terris J, Ecelbarger CA, Nielsen S, Knepper MA. Long-term regulation of four renal aquaporins in rats. Am J Physiol. 1996; 271:F414–F422. PMID: 8770174.
Article26. Hasler U, Mordasini D, Bens M, Bianchi M, Cluzeaud F, Rousselot M, Vandewalle A, Feraille E, Martin PY. Long term regulation of aquaporin-2 expression in vasopressin-responsive renal collecting duct principal cells. J Biol Chem. 2002; 277:10379–10386. PMID: 11782489.
Article27. Hasler U, Nielsen S, Féraille E, Martin PY. Posttranscriptional control of aquaporin-2 abundance by vasopressin in renal collecting duct principal cells. Am J Physiol Renal Physiol. 2006; 290:F177–F187. PMID: 15985652.
Article28. Nielsen S, Chou CL, Marples D, Christensen EI, Kishore BK, Knepper MA. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc Natl Acad Sci U S A. 1995; 92:1013–1017. PMID: 7532304.
Article29. Bustamante M, Hasler U, Kotova O, Chibalin AV, Mordasini D, Rousselot M, Vandewalle A, Martin PY, Féraille E. Insulin potentiates AVP-induced AQP2 expression in cultured renal collecting duct principal cells. Am J Physiol Renal Physiol. 2005; 288:F334–F344. PMID: 15494547.
Article30. Hasler U, Mordasini D, Bianchi M, Vandewalle A, Féraille E, Martin PY. Dual influence of aldosterone on AQP2 expression in cultured renal collecting duct principal cells. J Biol Chem. 2003; 278:21639–21648. PMID: 12660245.
Article31. Bustamante M, Hasler U, Leroy V, de Seigneux S, Dimitrov M, Mordasini D, Rousselot M, Martin PY, Féraille E. Calcium-sensing receptor attenuates AVP-induced aquaporin-2 expression via a calmodulin-dependent mechanism. J Am Soc Nephrol. 2008; 19:109–116. PMID: 18032798.32. Hasler U, Vinciguerra M, Vandewalle A, Martin PY, Féraille E. Dual effects of hypertonicity on aquaporin-2 expression in cultured renal collecting duct principal cells. J Am Soc Nephrol. 2005; 16:1571–1582. PMID: 15843469.
Article33. Nejsum LN. The renal plumbing system: aquaporin water channels. Cell Mol Life Sci. 2005; 62:1692–1706. PMID: 15924268.
Article34. Storm R, Klussmann E, Geelhaar A, Rosenthal W, Maric K. Osmolality and solute composition are strong regulators of AQP2 expression in renal principal cells. Am J Physiol Renal Physiol. 2003; 284:F189–F198. PMID: 12388395.35. Hasler U, Leroy V, Jeon US, Bouley R, Dimitrov M, Kim JA, Brown D, Kwon HM, Martin PY, Féraille E. NF-kappaB modulates aquaporin-2 transcription in renal collecting duct principal cells. J Biol Chem. 2008; 283:28095–28105. PMID: 18703515.36. Lee JH, Song MY, Song EK, Kim EK, Moon WS, Han MK, Park JW, Kwon KB, Park BH. Overexpression of SIRT1 protects pancreatic beta-cells against cytokine toxicity by suppressing the nuclear factor-kappaB signaling pathway. Diabetes. 2009; 58:344–351. PMID: 19008341.37. Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007; 100:1512–1521. PMID: 17446436.
Article38. Della-Morte D, Dave KR, DeFazio RA, Bao YC, Raval AP, Perez-Pinzon MA. Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway. Neuroscience. 2009; 159:993–1002. PMID: 19356683.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Resveratrol Supplementation on Oxidative Damage and Lipid Peroxidation Induced by Strenuous Exercise in Rats
- Resveratrol Protects HepG2 and Chang Liver Cells from Oxidative Stress
- Reduction of TNF alpha-induced oxidative DNA damage product, 8-hydroxy-2'-deoxyguanosine, in L929 cells stably transfected with small heat shock protein
- Dexamethasone enhances phospholipase D activity in M-1 cells
- The Influence of Antioxidant Resveratrol on Paraquat-induced Oxidative Stress in Cultured Lung Cells