Korean J Physiol Pharmacol.
2014 Apr;18(2):163-168. 10.4196/kjpp.2014.18.2.163.
Regular Exercise Training Increases the Number of Endothelial Progenitor Cells and Decreases Homocysteine Levels in Healthy Peripheral Blood
- Affiliations
-
- 1Department of Physiology, School of Medicine, Medical Research Institute, Pusan National University, Pusan National University, Yangsan 626-870, Korea. smkwon323@pusan.ac.kr
- 2Department of Surgery, Pusan National University Yangsan Hospital, Pusan National University, Yangsan 626-870, Korea.
- 3Immunoregulatory Therapeutics Group in Brain Busan 21 Project, Pusan National University, Yangsan 626-870, Korea.
- KMID: 2285506
- DOI: http://doi.org/10.4196/kjpp.2014.18.2.163
Abstract
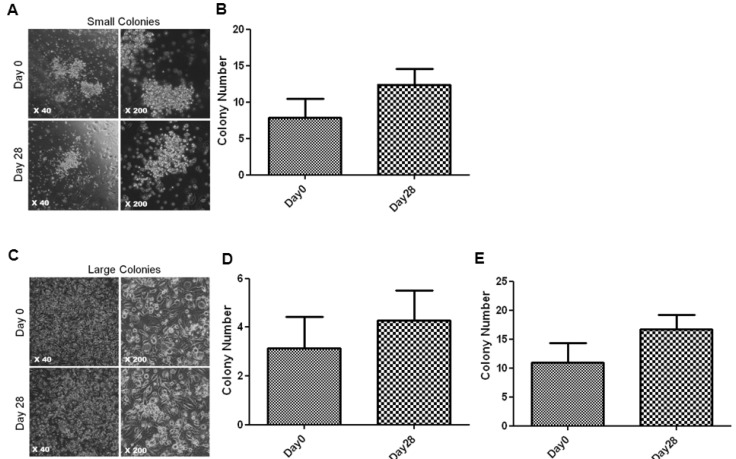
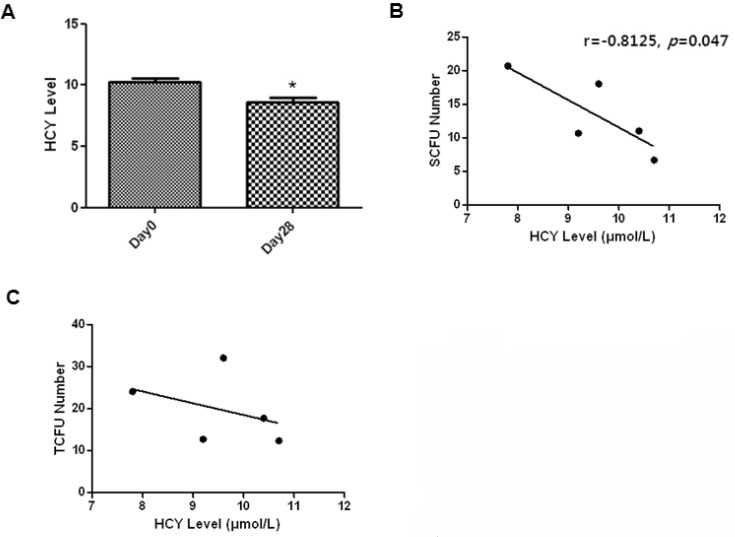
- Endothelial progenitor cells (EPCs) are known to play an important role in the repair of damaged blood vessels. We used an endothelial progenitor cell colony-forming assay (EPC-CFA) to determine whether EPC numbers could be increased in healthy individuals through regular exercise training. The number of functional EPCs obtained from human peripheral blood-derived AC133 stem cells was measured after a 28-day regular exercise training program. The number of total endothelial progenitor cell colony-forming units (EPC-CFU) was significantly increased compared to that in the control group (p=0.02, n=5). In addition, we observed a significant decrease in homocysteine levels followed by an increase in the number of EPC-CFUs (p=0.04, n=5), indicating that the 28-day regular exercise training could increase the number of EPC colonies and decrease homocysteine levels. Moreover, an inverse correlation was observed between small-endothelial progenitor cell colony-forming units (small-EPC-CFUs) and plasma homocysteine levels in healthy men (r=-0.8125, p=0.047). We found that regular exercise training could increase the number of EPC-CFUs and decrease homocysteine levels, thus decreasing the cardiovascular disease risk in men.
MeSH Terms
Figure
Reference
-
1. Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003; 348:593–600. PMID: 12584367.
Article2. Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001; 89:E1–E7. PMID: 11440984.
Article3. Möbius-Winkler S, Höllriegel R, Schuler G, Adams V. Endothelial progenitor cells: implications for cardiovascular disease. Cytometry A. 2009; 75:25–37. PMID: 19009636.
Article4. Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Böhm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005; 353:999–1007. PMID: 16148285.
Article5. Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002; 106:2781–2786. PMID: 12451003.
Article6. Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B, Rabelink TJ, van Zonneveld AJ. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004; 53:195–199. PMID: 14693715.7. Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, Kalka C. Impaired progenitor cell activity in age-related endothelial dysfunction. J Am Coll Cardiol. 2005; 45:1441–1448. PMID: 15862416.
Article8. Lenk K, Uhlemann M, Schuler G, Adams V. Role of endothelial progenitor cells in the beneficial effects of physical exercise on atherosclerosis and coronary artery disease. J Appl Physiol (1985). 2011; 111:321–328. PMID: 21350026.
Article9. Tsukada S, Kwon SM, Matsuda T, Jung SY, Lee JH, Lee SH, Masuda H, Asahara T. Identification of mouse colony-forming endothelial progenitor cells for postnatal neovascularization: a novel insight highlighted by new mouse colony-forming assay. Stem Cell Res Ther. 2013; 4:20. PMID: 23448126.
Article10. Manson JE, Greenland P, LaCroix AZ, Stefanick ML, Mouton CP, Oberman A, Perri MG, Sheps DS, Pettinger MB, Siscovick DS. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002; 347:716–725. PMID: 12213942.
Article11. Moebius-Winkler S, Schuler G, Adams V. Endothelial progenitor cells and exercise-induced redox regulation. Antioxid Redox Signal. 2011; 15:997–1011. PMID: 21091077.
Article12. Laufs U, Werner N, Link A, Endres M, Wassmann S, Jürgens K, Miche E, Böhm M, Nickenig G. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation. 2004; 109:220–226. PMID: 14691039.
Article13. Adams V, Lenk K, Linke A, Lenz D, Erbs S, Sandri M, Tarnok A, Gielen S, Emmrich F, Schuler G, Hambrecht R. Increase of circulating endothelial progenitor cells in patients with coronary artery disease after exercise-induced ischemia. Arterioscler Thromb Vasc Biol. 2004; 24:684–690. PMID: 14988094.
Article14. Möbius-Winkler S, Hilberg T, Menzel K, Golla E, Burman A, Schuler G, Adams V. Time-dependent mobilization of circulating progenitor cells during strenuous exercise in healthy individuals. J Appl Physiol (1985). 2009; 107:1943–1950. PMID: 19797690.
Article15. Zhu JH, Chen JZ, Wang XX, Xie XD, Sun J, Zhang FR. Homocysteine accelerates senescence and reduces proliferation of endothelial progenitor cells. J Mol Cell Cardiol. 2006; 40:648–652. PMID: 16600290.16. Alam MM, Mohammad AA, Shuaib U, Wang C, Ghani U, Schwindt B, Todd KG, Shuaib A. Homocysteine reduces endothelial progenitor cells in stroke patients through apoptosis. J Cereb Blood Flow Metab. 2009; 29:157–165. PMID: 18766198.
Article17. Chen JZ, Zhu JH, Wang XX, Zhu JH, Xie XD, Sun J, Shang YP, Guo XG, Dai HM, Hu SJ. Effects of homocysteine on number and activity of endothelial progenitor cells from peripheral blood. J Mol Cell Cardiol. 2004; 36:233–239. PMID: 14871551.18. Kwon YH, Jung SY, Kim JW, Lee SH, Lee JH, Lee BY, Kwon SM. Phloroglucinol inhibits the bioactivities of endothelial progenitor cells and suppresses tumor angiogenesis in LLC-tumor-bearing mice. PLoS One. 2012; 7:e33618. PMID: 22496756.
Article19. Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, Oh BH, Lee MM, Park YB. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004; 24:288–293. PMID: 14699017.
Article20. Fernandes T, Hashimoto NY, Schettert IT, Nakamuta JS, Krieger JE, Oliveira EMd. O grau de melhora na função das células progenitoras endoteliais derivadas da medula óssea é dependente do volume de treinamento físico aeróbio. Rev Bras Med Esporte. 2013; 19:260–266.
Article21. Randeva HS, Lewandowski KC, Drzewoski J, Brooke-Wavell K, O'Callaghan C, Czupryniak L, Hillhouse EW, Prelevic GM. Exercise decreases plasma total homocysteine in overweight young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002; 87:4496–4501. PMID: 12364425.
Article22. König D, Bissé E, Deibert P, Müller HM, Wieland H, Berg A. Influence of training volume and acute physical exercise on the homocysteine levels in endurance-trained men: interactions with plasma folate and vitamin B12. Ann Nutr Metab. 2003; 47:114–118. PMID: 12743461.23. Gelecek N, Teoman N, Ozdirenc M, Pinar L, Akan P, Bediz C, Kozan O. Influences of acute and chronic aerobic exercise on the plasma homocysteine level. Ann Nutr Metab. 2007; 51:53–58. PMID: 17356255.
Article24. Mano R, Ishida A, Ohya Y, Todoriki H, Takishita S. Dietary intervention with Okinawan vegetables increased circulating endothelial progenitor cells in healthy young women. Atherosclerosis. 2009; 204:544–548. PMID: 19013573.
Article25. Malinow MR. Hyperhomocyst(e)inemia. A common and easily reversible risk factor for occlusive atherosclerosis. Circulation. 1990; 81:2004–2006. PMID: 2188759.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Leptin on the Proliferation of the Endothelial Progenitor Cells from Peripheral Blood
- Adult Stem Cells: Beyond Regenerative Tool, More as a Bio-Marker in Obesity and Diabetes
- Association between Leukocyte Mitochondrial DNA Copy Number and Regular Exercise in Postmenopausal Women
- Effects of Exercise Training on Vascular Endothelial Function Related Factors of Obese Elderly Women with Sarcopenia
- Exercise in Patients with Advanced Diabetic Complications