Korean J Physiol Pharmacol.
2013 Dec;17(6):531-536. 10.4196/kjpp.2013.17.6.531.
Spontaneous Electrical Activity of Cultured Interstitial Cells of Cajal from Mouse Urinary Bladder
- Affiliations
-
- 1Department of Physiology, Chonnam National University Medical School, Gwangju 501-757, Korea. parkjs@jnu.ac.kr
- 2Department of Urology, Chonnam National University Medical School, Gwangju 501-757, Korea.
- 3Department of Neurosurgery, College of Medicine, Chosun University, Gwangju 501-759, Korea.
- 4Department of Physiology, College of Medicine, Chosun University, Gwangju 501-759, Korea.
- 5Research Institute of Medical Sciences, Chonnam National University, Gwangju 501-757, Korea.
- 6Center for Creative Biomedical Scientists at Chonnam National Univertisity, Gwangju 501-757, Korea.
- KMID: 2285481
- DOI: http://doi.org/10.4196/kjpp.2013.17.6.531
Abstract
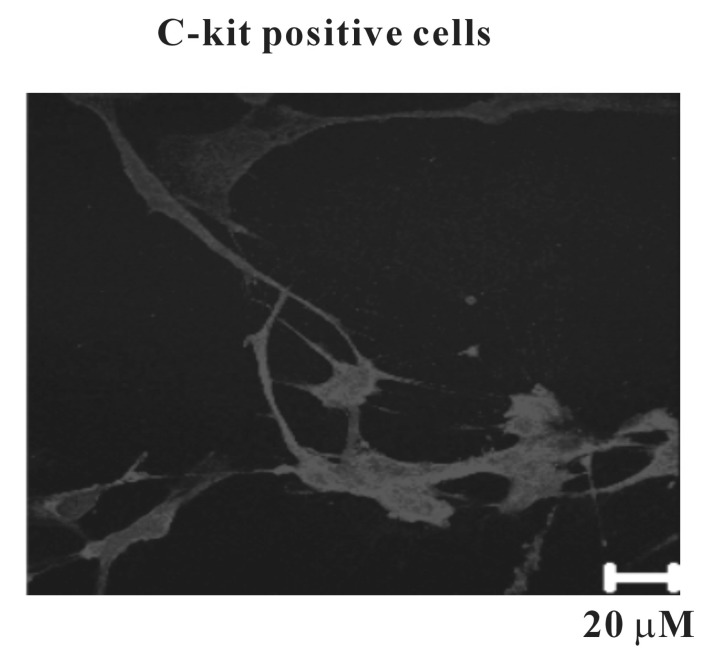
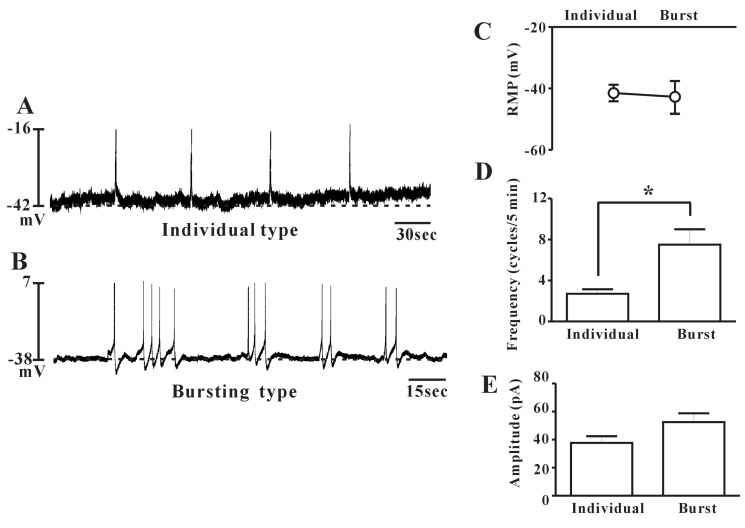
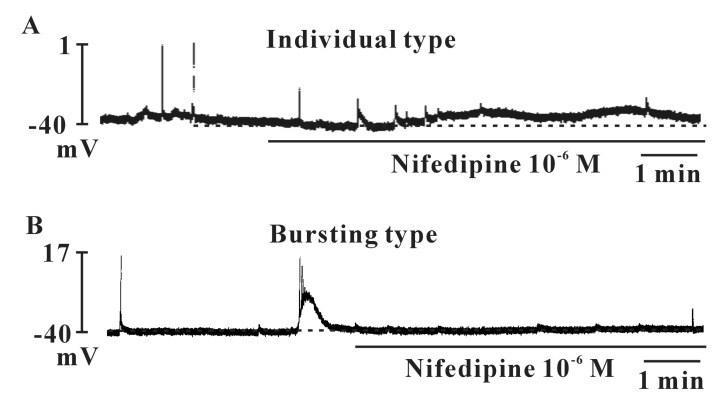
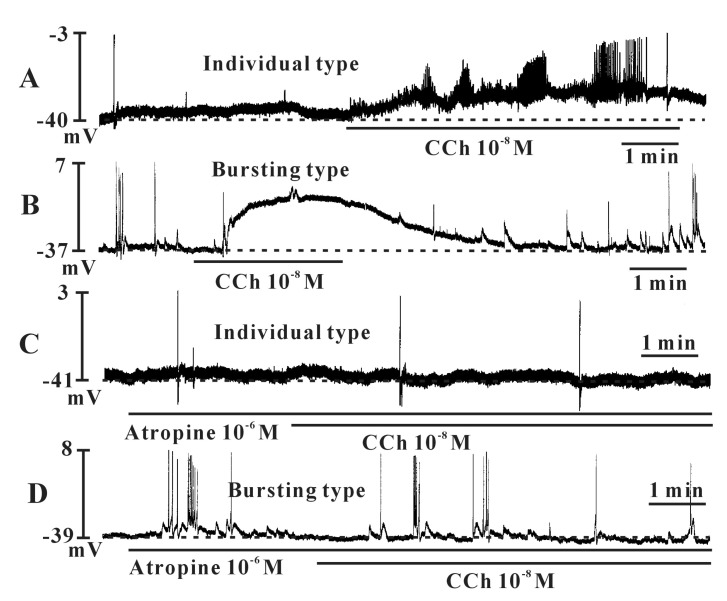
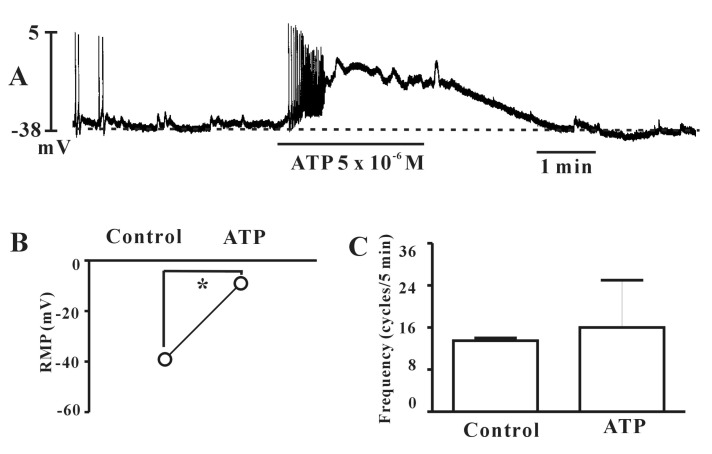
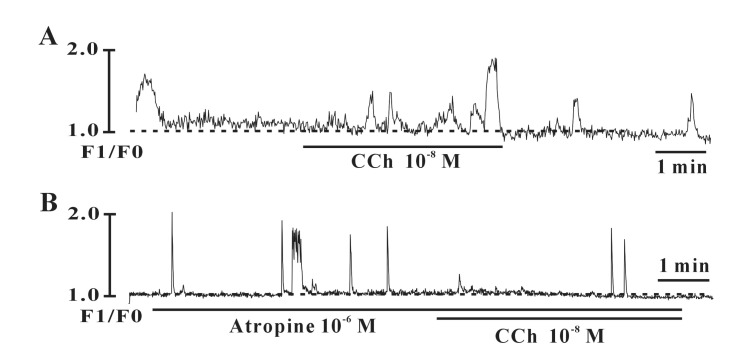
- Interstitial cells of Cajal (ICCs) from the urinary bladder regulate detrusor smooth muscle activities. We cultured ICCs from the urinary bladder of mice and performed patch clamp and intracellular Ca2+ ([Ca2+]i) imaging to investigate whether cultured ICCs can be a valuable tool for cellular functional studies. The cultured ICCs displayed two types of spontaneous electrical activities which are similar to those recorded in intact bladder tissues. Spontaneous electrical activities of cultured ICCs were nifedipine-sensitive. Carbachol and ATP, both excitatory neurotransmitters in the urinary bladder, depolarized the membrane and increased the frequency of spike potentials. Carbachol increased [Ca2+]i oscillations and basal Ca2+ levels, which were blocked by atropine. These results suggest that cultured ICCs from the urinary bladder retain rhythmic phenotypes similar to the spontaneous electrical activities recorded from the intact urinary bladder. Therefore, we suggest that cultured ICCs from the urinary bladder may be useful for cellular and molecular studies of ICCs.
MeSH Terms
Figure
Reference
-
1. Thomsen L, Robinson TL, Lee JC, Farraway LA, Hughes MJ, Andrews DW, Huizinga JD. Interstitial cells of Cajal generate a rhythmic pacemaker current. Nat Med. 1998; 4:848–851. PMID: 9662380.
Article2. Sanders KM, Koh SD, Ward SM. Interstitial cells of cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006; 68:307–343. PMID: 16460275.
Article3. Thornbury KD, Hollywood MA, McHale NG, Sergeant GP. Cajal beyond the gut: interstitial cells in the urinary system--towards general regulatory mechanisms of smooth muscle contractility? Acta Gastroenterol Belg. 2011; 74:536–542. PMID: 22319963.4. McCloskey KD. Interstitial cells of Cajal in the urinary tract. Handb Exp Pharmacol. 2011; (202):233–254. PMID: 21290229.
Article5. Davidson RA, McCloskey KD. Morphology and localization of interstitial cells in the guinea pig bladder: structural relationships with smooth muscle and neurons. J Urol. 2005; 173:1385–1390. PMID: 15758810.
Article6. Lagou M, De Vente J, Kirkwood TB, Hedlund P, Andersson KE, Gillespie JI, Drake MJ. Location of interstitial cells and neurotransmitters in the mouse bladder. BJU Int. 2006; 97:1332–1337. PMID: 16686734.
Article7. Min Y, He P, Wang Q, Jin X, Song B, Li L. The effects of the c-kit blocker glivec on the contractile response of urinary bladder. J Surg Res. 2011; 171:e193–e199. PMID: 21962730.
Article8. Vahabi B, McKay NG, Lawson K, Sellers DJ. The role of c-kit-positive interstitial cells in mediating phasic contractions of bladder strips from streptozotocin-induced diabetic rats. BJU Int. 2011; 107:1480–1487. PMID: 20735390.
Article9. Grol S, Essers PB, van Koeveringe GA, Martinez-Martinez P, de Vente J, Gillespie JI. M(3) muscarinic receptor expression on suburothelial interstitial cells. BJU Int. 2009; 104:398–405. PMID: 19338557.10. Wang Y, Fang Q, Lu Y, Song B, Li W, Li L. Effects of mechanical stretch on interstitial cells of Cajal in guinea pig bladder. J Surg Res. 2010; 164:e213–e219. PMID: 20828727.
Article11. Kubota Y, Hashitani H, Shirasawa N, Kojima Y, Sasaki S, Mabuchi Y, Soji T, Suzuki H, Kohri K. Altered distribution of interstitial cells in the guinea pig bladder following bladder outlet obstruction. Neurourol Urodyn. 2008; 27:330–340. PMID: 17724735.
Article12. Ward SM, Ordog T, Koh SD, Baker SA, Jun JY, Amberg G, Monaghan K, Sanders KM. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J Physiol. 2000; 525 Pt 2:355–361. PMID: 10835039.
Article13. Nakayama S, Ohya S, Liu HN, Watanabe T, Furuzono S, Wang J, Nishizawa Y, Aoyama M, Murase N, Matsubara T, Ito Y, Imaizumi Y, Kajioka S. Sulphonylurea receptors differently modulate ICC pacemaker Ca2+ activity and smooth muscle contractility. J Cell Sci. 2005; 118:4163–4173. PMID: 16141235.14. Choi S, Yeum CH, Kim YD, Park CG, Kim MY, Park JS, Jeong HS, Kim BJ, So I, Kim KW. Receptor tyrosine and MAP kinase are involved in effects of H2O2 on interstitial cells of Cajal in murine intestine. J Cell Mol Med. 2010; 14:257–266. PMID: 20414970.15. Anderson UA, Carson C, McCloskey KD. KCNQ currents and their contribution to resting membrane potential and the excitability of interstitial cells of Cajal from the guinea pig bladder. J Urol. 2009; 182:330–336. PMID: 19450820.
Article16. Deng J, He P, Zhong X, Wang Q, Li L, Song B. Identification of T-type calcium channels in the interstitial cells of Cajal in rat bladder. Urology. 2012; 80:1389.e1. 1389.e7. PMID: 22995572.
Article17. McCloskey KD. Characterization of outward currents in interstitial cells from the guinea pig bladder. J Urol. 2005; 173:296–301. PMID: 15592100.
Article18. Shahi PK, Choi S, Zuo DC, Yeum CH, Yoon PJ, Lee J, Kim YD, Park CG, Kim MY, Shin HR, Oh HJ, Jun JY. 5-hydroxytryptamine generates tonic inward currents on pacemaker activity of interstitial cells of cajal from mouse small intestine. Korean J Physiol Pharmacol. 2011; 15:129–135. PMID: 21860590.
Article19. Kim SO, Oh BS, Chang IY, Song SH, Ahn K, Hwang EC, Oh KJ, Kwon D, Park K. Distribution of interstitial cells of Cajal and expression of nitric oxide synthase after experimental bladder outlet obstruction in a rat model of bladder overactivity. Neurourol Urodyn. 2011; 30:1639–1645. PMID: 21780165.
Article20. Hashitani H, Brading AF. Electrical properties of detrusor smooth muscles from the pig and human urinary bladder. Br J Pharmacol. 2003; 140:146–158. PMID: 12967944.
Article21. Hashitani H, Brading AF, Suzuki H. Correlation between spontaneous electrical, calcium and mechanical activity in detrusor smooth muscle of the guinea-pig bladder. Br J Pharmacol. 2004; 141:183–193. PMID: 14662721.
Article22. Hashitani H, Brading AF. Ionic basis for the regulation of spontaneous excitation in detrusor smooth muscle cells of the guinea-pig urinary bladder. Br J Pharmacol. 2003; 140:159–169. PMID: 12967945.
Article23. McCloskey KD. Calcium currents in interstitial cells from the guinea-pig bladder. BJU Int. 2006; 97:1338–1343. PMID: 16686735.
Article24. Finney SM, Stewart LH, Gillespie JI. Cholinergic activation of phasic activity in the isolated bladder: possible evidence for M3- and M2-dependent components of a motor/sensory system. BJU Int. 2007; 100:668–678. PMID: 17627783.
Article25. O'Reilly BA, Kosaka AH, Chang TK, Ford AP, Popert R, McMahon SB. A quantitative analysis of purinoceptor expression in the bladders of patients with symptomatic outlet obstruction. BJU Int. 2001; 87:617–622. PMID: 11350400.26. Burnstock G. Purinergic signalling in the lower urinary tract. Acta Physiol (Oxf). 2013; 207:40–52. PMID: 23176070.
Article27. Badawi JK, Seja T, Uecelehan H, Honeck P, Kwon ST, Bross S, Langbein S. Relaxation of human detrusor muscle by selective beta-2 and beta-3 agonists and endogenous catecholamines. Urology. 2007; 69:785–790. PMID: 17445682.
Article28. Yanai Y, Hashitani H, Hayase M, Sasaki S, Suzuki H, Kohri K. Role of nitric oxide/cyclic GMP pathway in regulating spontaneous excitations in detrusor smooth muscle of the guinea-pig bladder. Neurourol Urodyn. 2008; 27:446–453. PMID: 17929303.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Modulation of Pacemaker Ca2+ Activity by Serotonin in the Cultured Interstitial Cells of the Cajal Clusters Isolated from Mouse Small Intestine
- Capsaicin Inhibits the Spontaneous Pacemaker Activity in Interstitial Cells of Cajal From the Small Intestine of Mouse
- Vanilloid Receptor Type-1 Immunoreactivities in the Mouse Myenteric Plexus: Immunohistochemical and Electrophysiological Study
- Distribution of Interstitial Cells of Cajal in Menopausal Rat Urinary Bladder Showing Detrusor Overactivity
- Regulation of Intracellular Calcium by Endoplasmic Reticulum Proteins in Small Intestinal Interstitial Cells of Cajal