Korean J Physiol Pharmacol.
2013 Oct;17(5):463-468. 10.4196/kjpp.2013.17.5.463.
Disappearance of Hypoxic Pulmonary Vasoconstriction and O2-Sensitive Nonselective Cationic Current in Arterial Myocytes of Rats Under Ambient Hypoxia
- Affiliations
-
- 1Department of Physiology and Ischemic/Hypoxic Disease Institute, Seoul National University College of Medicine, Seoul 110-799, Korea. sjoonkim@snu.ac.kr
- KMID: 1500243
- DOI: http://doi.org/10.4196/kjpp.2013.17.5.463
Abstract
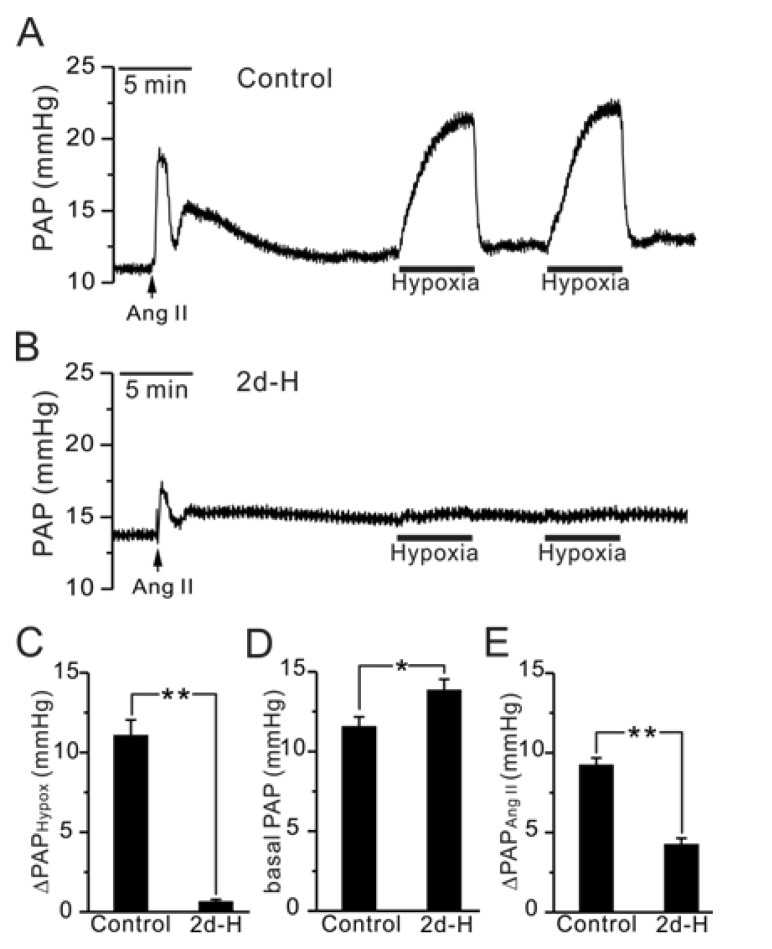
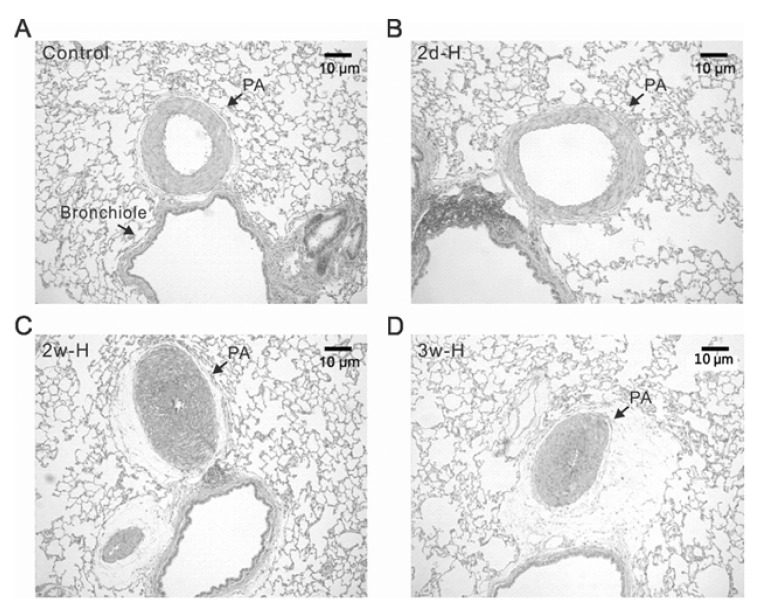
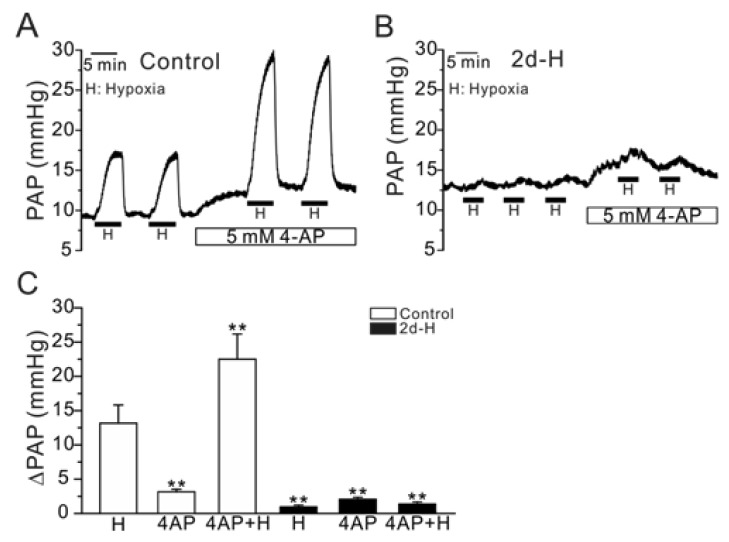
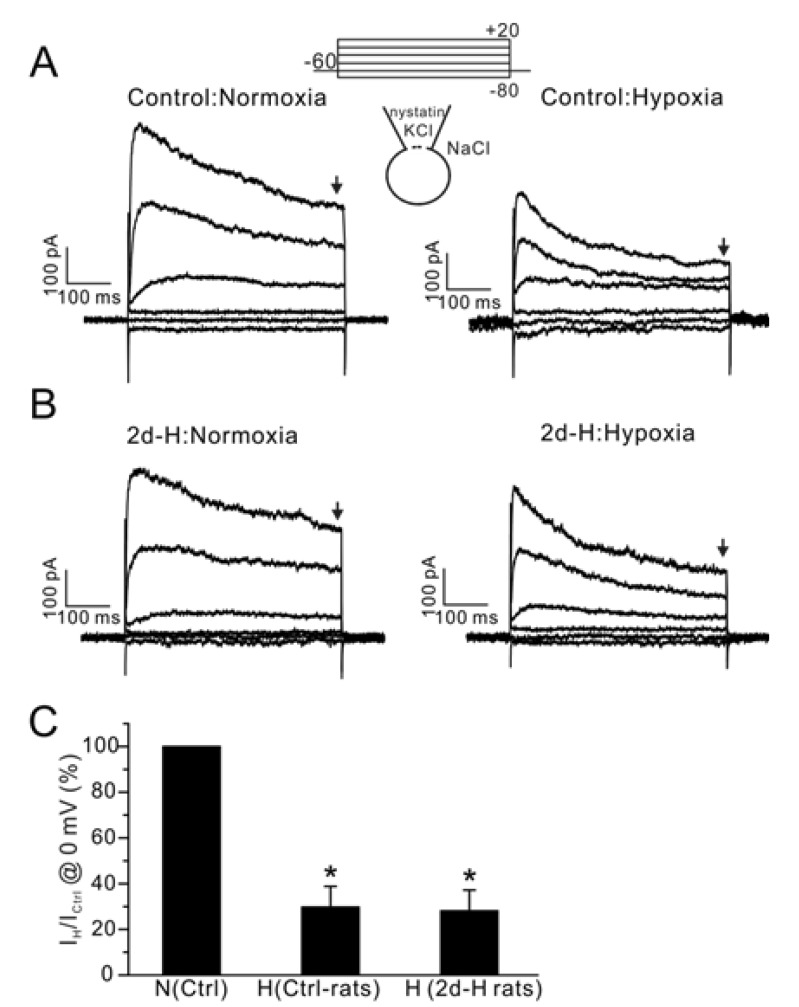
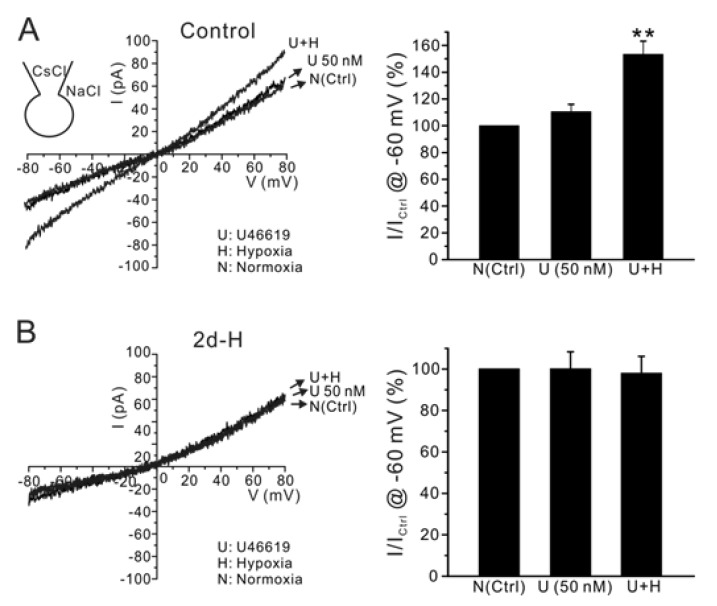
- Acute hypoxia induces contraction of pulmonary artery (PA) to protect ventilation/perfusion mismatch in lungs. As for the cellular mechanism of hypoxic pulmonary vasoconstriction (HPV), hypoxic inhibition of voltage-gated K+ channel (Kv) in PA smooth muscle cell (PASMC) has been suggested. In addition, our recent study showed that thromboxane A2 (TXA2) and hypoxia-activated nonselective cation channel (I(NSC)) is also essential for HPV. However, it is not well understood whether HPV is maintained in the animals exposed to ambient hypoxia for two days (2d-H). Specifically, the associated electrophysiological changes in PASMCs have not been studied. Here we investigate the effects of 2d-H on HPV in isolated ventilated/perfused lungs (V/P lungs) from rats. HPV was almost abolished without structural remodeling of PA in 2d-H rats, and the lost HPV was not recovered by Kv inhibitor, 4-aminopyridine. Patch clamp study showed that the hypoxic inhibition of Kv current in PASMC was similar between 2d-H and control. In contrast, hypoxia and TXA2-activated I(NSC) was not observed in PASMCs of 2d-H. From above results, it is suggested that the decreased I(NSC) might be the primary functional cause of HPV disappearance in the relatively early period (2 d) of hypoxia.
Keyword
MeSH Terms
Figure
Reference
-
1. Weir EK, Archer SL. The mechanism of acute hypoxic pulmonary vasoconstriction: the tale of two channels. FASEB J. 1995; 9:183–189. PMID: 7781921.
Article2. Archer SL, Wu XC, Thébaud B, Nsair A, Bonnet S, Tyrrell B, McMurtry MS, Hashimoto K, Harry G, Michelakis ED. Preferential expression and function of voltage-gated, O2-sensitive K+ channels in resistance pulmonary arteries explains regional heterogeneity in hypoxic pulmonary vasoconstriction: ionic diversity in smooth muscle cells. Circ Res. 2004; 95:308–318. PMID: 15217912.3. Osipenko ON, Tate RJ, Gurney AM. Potential role for kv3.1b channels as oxygen sensors. Circ Res. 2000; 86:534–540. PMID: 10720415.
Article4. Sweeney M, Yuan JX. Hypoxic pulmonary vasoconstriction: role of voltage-gated potassium channels. Respir Res. 2000; 1:40–48. PMID: 11667964.
Article5. Yoo HY, Park SJ, Seo EY, Park KS, Han JA, Kim KS, Shin DH, Earm YE, Zhang YH, Kim SJ. Role of thromboxane A2-activated nonselective cation channels in hypoxic pulmonary vasoconstriction of rat. Am J Physiol Cell Physiol. 2012; 302:C307–C317. PMID: 21998141.6. Pierson DJ. Pathophysiology and clinical effects of chronic hypoxia. Respir Care. 2000; 45:39–51. discussion 51-53. PMID: 10771781.7. Pozeg ZI, Michelakis ED, McMurtry MS, Thébaud B, Wu XC, Dyck JR, Hashimoto K, Wang S, Moudgil R, Harry G, Sultanian R, Koshal A, Archer SL. In vivo gene transfer of the O2-sensitive potassium channel Kv1.5 reduces pulmonary hypertension and restores hypoxic pulmonary vasoconstriction in chronically hypoxic rats. Circulation. 2003; 107:2037–2044. PMID: 12695303.8. Reeve HL, Michelakis E, Nelson DP, Weir EK, Archer SL. Alterations in a redox oxygen sensing mechanism in chronic hypoxia. J Appl Physiol (1985). 2001; 90:2249–2256. PMID: 11356790.
Article9. McMurtry IF, Petrun MD, Reeves JT. Lungs from chronically hypoxic rats have decreased pressor response to acute hypoxia. Am J Physiol. 1978; 235:H104–H109. PMID: 677322.
Article10. Asano K, Yanagidaira Y, Yoshimura K, Sakai A. The cGMP pathway is not responsible for the blunted hypoxic vasoconstriction in rat lungs after altitude exposure. Acta Physiol Scand. 1997; 160:393–400. PMID: 9338521.
Article11. Moudgil R, Michelakis ED, Archer SL. The role of K+ channels in determining pulmonary vascular tone, oxygen sensing, cell proliferation, and apoptosis: implications in hypoxic pulmonary vasoconstriction and pulmonary arterial hypertension. Microcirculation. 2006; 13:615–632. PMID: 17085423.12. Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res. 2006; 99:675–691. PMID: 17008597.13. Laursen BE, Dam MY, Mulvany MJ, Simonsen U. Hypoxia-induced pulmonary vascular remodeling and right ventricular hypertrophy is unaltered by long-term oral L-arginine administration. Vascul Pharmacol. 2008; 49:71–76. PMID: 18499529.
Article14. Weissmann N, Nollen M, Gerigk B, Ardeschir Ghofrani H, Schermuly RT, Gunther A, Quanz K, Fink L, Hänze J, Rose F, Seeger W, Grimminger F. Downregulation of hypoxic vasoconstriction by chronic hypoxia in rabbits: effects of nitric oxide. Am J Physiol Heart Circ Physiol. 2003; 284:H931–H938. PMID: 12433654.15. Yuan JX. Oxygen-sensitive K+ channel(s): where and what? Am J Physiol Lung Cell Mol Physiol. 2001; 281:L1345–L1349. PMID: 11704529.16. Park SJ, Yoo HY, Kim HJ, Kim JK, Zhang YH, Kim SJ. Requirement of pretone by thromboxane a2 for hypoxic pulmonary vasoconstriction in precision-cut lung slices of rat. Korean J Physiol Pharmacol. 2012; 16:59–64. PMID: 22416221.17. Peng G, Lu W, Li X, Chen Y, Zhong N, Ran P, Wang J. Expression of store-operated Ca2+ entry and transient receptor potential canonical and vanilloid-related proteins in rat distal pulmonary venous smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2010; 299:L621–L630. PMID: 20693314.18. Weissmann N, Dietrich A, Fuchs B, Kalwa H, Ay M, Dumitrascu R, Olschewski A, Storch U, Mederos y Schnitzler M, Ghofrani HA, Schermuly RT, Pinkenburg O, Seeger W, Grimminger F, Gudermann T. Classical transient receptor potential channel 6 (TRPC6) is essential for hypoxic pulmonary vasoconstriction and alveolar gas exchange. Proc Natl Acad Sci U S A. 2006; 103:19093–19098. PMID: 17142322.
Article19. Yu Y, Fantozzi I, Remillard CV, Landsberg JW, Kunichika N, Platoshyn O, Tigno DD, Thistlethwaite PA, Rubin LJ, Yuan JX. Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc Natl Acad Sci U S A. 2004; 101:13861–13866. PMID: 15358862.
Article20. Urban N, Hill K, Wang L, Kuebler WM, Schaefer M. Novel pharmacological TRPC inhibitors block hypoxia-induced vasoconstriction. Cell Calcium. 2012; 51:194–206. PMID: 22280812.
Article21. Fagan KA, Oka M, Bauer NR, Gebb SA, Ivy DD, Morris KG, McMurtry IF. Attenuation of acute hypoxic pulmonary vasoconstriction and hypoxic pulmonary hypertension in mice by inhibition of Rho-kinase. Am J Physiol Lung Cell Mol Physiol. 2004; 287:L656–L664. PMID: 14977625.
Article22. Naeije R, Barberà JA. Pulmonary hypertension associated with COPD. Crit Care. 2001; 5:286–289. PMID: 11737906.23. Zubieta-Castillo G Sr, Zubieta-Calleja GR Jr, Zubieta-Calleja L. Chronic mountain sickness: the reaction of physical disorders to chronic hypoxia. J Physiol Pharmacol. 2006; 57(Suppl 4):431–442. PMID: 17072074.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hypoxic Pulmonary Vasoconstriction and Change in Outward K Current in Pulmonary Arterial Smooth Muscle Cell of the Rabbit
- Changes of Hypoxic Pulmonary Vasoconstriction in Isolated Endotoxin-Treated Rat Lungs
- Effects of Glibenclamide and L-NAME on Hypoxic Pulmonary Vasoconstriction in Rats
- Pathophysiology of Persistent Pulmonary Hypertension of the Newborn
- Influence of Changes in Mixed Venous Oxygen Tension(PvO2) by Extracorporeal Membrane Oxygenation(ECMO) on Hypoxic Pulmonary Vasoconstriction