Korean J Physiol Pharmacol.
2012 Feb;16(1):25-30. 10.4196/kjpp.2012.16.1.25.
Bile Acid Inhibition of N-type Calcium Channel Currents from Sympathetic Ganglion Neurons
- Affiliations
-
- 1Department of Pharmacology, University of Ulsan College of Medicine, Seoul 138-736, Korea. kyung@amc.seoul.kr
- 2Laboratory for Craniofacial Research, Chonbuk National University Dental School, Jeonju 561-756, Korea.
- KMID: 2285436
- DOI: http://doi.org/10.4196/kjpp.2012.16.1.25
Abstract
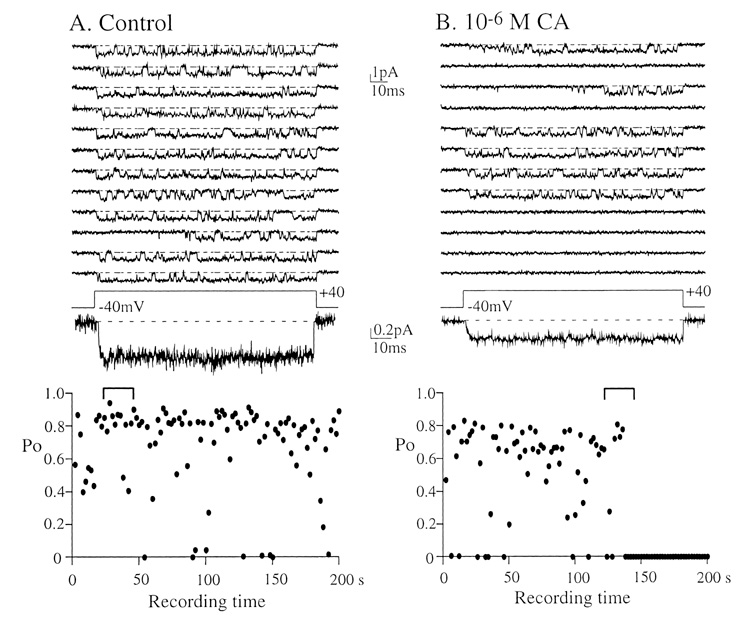
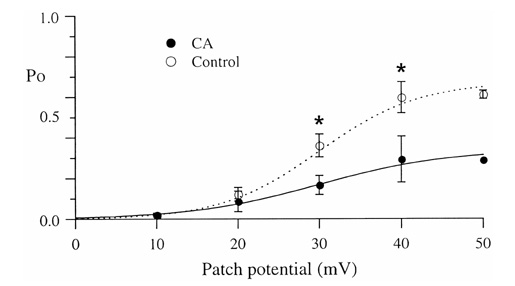
- Under some pathological conditions as bile flow obstruction or liver diseases with the enterohepatic circulation being disrupted, regurgitation of bile acids into the systemic circulation occurs and the plasma level of bile acids increases. Bile acids in circulation may affect the nervous system. We examined this possibility by studying the effects of bile acids on gating of neuronal (N)-type Ca2+ channel that is essential for neurotransmitter release at synapses of the peripheral and central nervous system. N-type Ca2+ channel currents were recorded from bullfrog sympathetic neuron under a cell-attached mode using 100 mM Ba2+ as a charge carrier. Cholic acid (CA, 10(-6) M) that is relatively hydrophilic thus less cytotoxic was included in the pipette solution. CA suppressed the open probability of N-type Ca2+ channel, which appeared to be due to an increase in null (no activity) sweeps. For example, the proportion of null sweep in the presence of CA was ~40% at +40 mV as compared with ~8% in the control recorded without CA. Other single channel properties including slope conductance, single channel current amplitude, open and shut times were not significantly affected by CA being present. The results suggest that CA could modulate N-type Ca2+ channel gating at a concentration as low as 10(-6) M. Bile acids have been shown to activate nonselective cation conductance and depolarize the cell membrane. Under pathological conditions with increased circulating bile acids, CA suppression of N-type Ca2+ channel function may be beneficial against overexcitation of the synapses.
MeSH Terms
-
Bile
Bile Acids and Salts
Calcium Channels, N-Type
Cell Membrane
Central Nervous System
Cholic Acid
Enterohepatic Circulation
Fees and Charges
Ganglia, Sympathetic
Liver Diseases
Nervous System
Neurons
Neurotransmitter Agents
Plasma
Rana catesbeiana
Synapses
Bile Acids and Salts
Calcium Channels, N-Type
Cholic Acid
Neurotransmitter Agents
Figure
Reference
-
1. Borgstrom B, Barrowman JA, Lindstrom M. Danielsson H, Sjovall J, editors. Roles of bile acids in intestinal lipid digestion in absorption. Sterols and bile acids. 1985. Amsterdam: Elsevir;405–425.2. Hofmann AF. Chemistry and enterohepatic circulation of bile acids. Hepatology. 1984. 4:5 Suppl. 4S–14S.3. Osuga T, Mitamura K, Mashige F, Imai D. Evaluation of fluorimetrically estimated serum bile acid in liver disease. Clin Chim Acta. 1977. 75:81–90.4. Hofmann AF. Bile acids: the good, the bad, and the ugly. News Physiol Sci. 1999. 14:24–29.5. Shah N, Khurana S, Cheng K, Raufman JP. Muscarinic receptors and ligands in cancer. Am J Physiol Cell Physiol. 2009. 296:C221–C232.6. Patti ME, Houten SM, Bianco AC, Bernier R, Larsen PR, Holst JJ, Badman MK, Maratos-Flier E, Mun EC, Pihlajamaki J, Auwerx J, Goldfine AB. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring). 2009. 17:1671–1677.7. Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, Pellicciari R, Auwerx J, Schoonjans K. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009. 10:167–177.8. Jansen PL. A new life for bile acids. J Hepatol. 2010. 52:937–938.9. Thomas C, Auwerx J, Schoonjans K. Bile acids and the membrane bile acid receptor TGR5--connecting nutrition and metabolism. Thyroid. 2008. 18:167–174.10. Khurana S, Raufman JP, Pallone TL. Bile acids regulate cardiovascular function. Clin Transl Sci. 2011. 4:210–218.11. Jun JY, Choi S, Chang IY, Yoon CK, Jeong HG, Kong ID, So I, Kim KW, You HJ. Deoxycholic acid inhibits pacemaker currents by activating ATP-dependent K+ channels through prostaglandin E2 in interstitial cells of Cajal from the murine small intestine. Br J Pharmacol. 2005. 144:242–251.12. Raufman JP, Cheng K, Zimniak P. Activation of muscarinic receptor signaling by bile acids: physiological and medical implications. Dig Dis Sci. 2003. 48:1431–1444.13. Maruyama T, Miyamoto Y, Nakamura T, Tamai Y, Okada H, Sugiyama E, Nakamura T, Itadani H, Tanaka K. Identification of membrane-type receptor for bile acids (M-BAR). Biochem Biophys Res Commun. 2002. 298:714–719.14. Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y, Hinuma S, Fujisawa Y, Fujino M. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003. 278:9435–9440.15. Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science. 1999. 284:1362–1365.16. Zimber A, Gespach C. Bile acids and derivatives, their nuclear receptors FXR, PXR and ligands: role in health and disease and their therapeutic potential. Anticancer Agents Med Chem. 2008. 8:540–563.17. Hylemon PB, Zhou H, Pandak WM, Ren S, Gil G, Dent P. Bile acids as regulatory molecules. . J Lipid Res. 2009. 50:1509–1520.18. Kostenis E. A glance at G-protein-coupled receptors for lipid mediators: a growing receptor family with remarkably diverse ligands. Pharmacol Ther. 2004. 102:243–257.19. Tiwari A, Maiti P. TGR5: an emerging bile acid G-protein-coupled receptor target for the potential treatment of metabolic disorders. Drug Discov Today. 2009. 14:523–530.20. Dopico AM, Walsh JV Jr, Singer JJ. Natural bile acids and synthetic analogues modulate large conductance Ca2+-activated K+ (BKCa) channel activity in smooth muscle cells. J Gen Physiol. 2002. 119:251–273.21. Hirning LD, Fox AP, McCleskey EW, Olivera BM, Thayer SA, Miller RJ, Tsien RW. Dominant role of N-type Ca2+ channels in evoked release of norepinephrine from sympathetic neurons. Science. 1988. 239:57–61.22. Lee HK, Elmslie KS. Gating of single N-type calcium channels recorded from bullfrog sympathetic neurons. J Gen Physiol. 1999. 113:111–124.23. Heuman DM. Quantitative estimation of the hydrophilichydrophobic balance of mixed bile salt solutions. J Lipid Res. 1989. 30:719–730.24. Bomzon A, Ljubuncic P. Bile acids as endogenous vasodilators? Biochem Pharmacol. 1995. 49:581–589.25. Sharma R, Majer F, Peta VK, Wang J, Keaveney R, Kelleher D, Long A, Gilmer JF. Bile acid toxicity structure-activity relationships: correlations between cell viability and lipophilicity in a panel of new and known bile acids using an oesophageal cell line (HET-1A). Bioorg Med Chem. 2010. 18:6886–6895.26. Weltzien HU. Cytolytic and membrane-perturbing properties of lysophosphatidylcholine. Biochim Biophys Acta. 1979. 559:259–287.27. Posa M, Kuhajda K. Hydrophobicity and haemolytic potential of oxo derivatives of cholic, deoxycholic and chenodeoxycholic acids. Steroids. 2010. 75:424–431.28. Roda A, Hofmann AF, Mysels KJ. The influence of bile salt structure on self-association in aqueous solutions. J Biol Chem. 1983. 258:6362–6370.29. Posa M, Kevresan S, Mikov M, Cirin-Novta V, Sârbu C, Kuhajda K. Determination of critical micellar concentrations of cholic acid and its keto derivatives. Colloids Surf B Biointerfaces. 2007. 59:179–183.30. Yang L, Zhang H, Mikov M, Tucker IG. Physicochemical and biological characterization of monoketocholic acid, a novel permeability enhancer. Mol Pharm. 2009. 6:448–456.31. Roda A, Cappelleri G, Aldini R, Roda E, Barbara L. Quantitative aspects of the interaction of bile acids with human serum albumin. J Lipid Res. 1982. 23:490–495.32. Pico GA, Houssier C. Bile salts-bovine serum albumin binding: spectroscopic and thermodynamic studies. Biochim Biophys Acta. 1989. 999:128–134.33. Ceryak S, Bouscarel B, Fromm H. Comparative binding of bile acids to serum lipoproteins and albumin. J Lipid Res. 1993. 34:1661–1674.34. Ikeda SR. Voltage-dependent modulation of N-type calcium channels by G-protein beta gamma subunits. Nature. 1996. 380:255–258.35. Lee HK, Elmslie KS. Reluctant gating of single N-type calcium channels during neurotransmitter-induced inhibition in bullfrog sympathetic neurons. J Neurosci. 2000. 20:3115–3128.36. Lundbaek JA, Birn P, Girshman J, Hansen AJ, Andersen OS. Membrane stiffness and channel function. Biochemistry. 1996. 35:3825–3830.37. Klemic KG, Shieh CC, Kirsch GE, Jones SW. Inactivation of Kv2.1 potassium channels. Biophys J. 1998. 74:1779–1789.38. Patil PG, Brody DL, Yue DT. Preferential closed-state inactivation of neuronal calcium channels. Neuron. 1998. 20:1027–1038.39. Dunlap K, Luebke JI, Turner TJ. Exocytotic Ca2+ channels in mammalian central neurons. Trends Neurosci. 1995. 18:89–98.40. Wu LG, Saggau P. Presynaptic inhibition of elicited neurotransmitter release. Trends Neurosci. 1997. 20:204–212.41. Jeon D, Kim C, Yang YM, Rhim H, Yim E, Oh U, Shin HS. Impaired long-term memory and long-term potentiation in N-type Ca2+ channel-deficient mice. Genes Brain Behav. 2007. 6:375–388.42. Gross RA, Macdonald RL. Dynorphin A selectively reduces a large transient (N-type) calcium current of mouse dorsal root ganglion neurons in cell culture. Proc Natl Acad Sci USA. 1987. 84:5469–5473.43. Scroggs RS, Fox AP. Multiple Ca2+ currents elicited by action potential waveforms in acutely isolated adult rat dorsal root ganglion neurons. J Neurosci. 1992. 12:1789–1801.44. Bowersox SS, Gadbois T, Singh T, Pettus M, Wang YX, Luther RR. Selective N-type neuronal voltage-sensitive calcium channel blocker, SNX-111, produces spinal antinociception in rat models of acute, persistent and neuropathic pain. J Pharmacol Exp Ther. 1996. 279:1243–1249.45. Liu L, Zwingman TA, Fletcher CF. In vivo analysis of voltage-dependent calcium channels. J Bioenerg Biomembr. 2003. 35:671–685.46. Ino M, Yoshinaga T, Wakamori M, Miyamoto N, Takahashi E, Sonoda J, Kagaya T, Oki T, Nagasu T, Nishizawa Y, Tanaka I, Imoto K, Aizawa S, Koch S, Schwartz A, Niidome T, Sawada K, Mori Y. Functional disorders of the sympathetic nervous system in mice lacking the alpha 1B subunit (Cav 2.2) of N-type calcium channels. Proc Natl Acad Sci USA. 2001. 98:5323–5328.47. Devor DC, Sekar MC, Frizzell RA, Duffey ME. Taurodeoxycholate activates potassium and chloride conductances via an IP3-mediated release of calcium from intracellular stores in a colonic cell line (T84). J Clin Invest. 1993. 92:2173–2181.48. Lee HK, Lee KH. Bile acid modulation of gastroinstinal smooth muscle contraction and ionic currents. Korean J Physiol Pharmacol. 2000. 4:333–338.49. Wehner F. Taurocholate depolarizes rat hepatocytes in primary culture by increasing cell membrane Na+ conductance. Pflugers Arch. 1993. 424:145–151.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nimodipine as a potential pharmacological tool for characterizing R-type calcium currents
- Effect of Fluoxetine on Calcium or Potassium Channels in the Neuron of Rat Major Pelvic Ganglia
- The Effect of DA-5018, A Synthetic Capsaicin Derivative,on Voltage-Dependent Calcium Currents in TrigeminalGanglion Neurons of Neonatal Rats
- N-type calcium channels
- Identification of ATP-sensitive K+ Conductances in Male Rat Major Pelvic Ganglion Neurons