Increase in the Prevalence of Carbapenem-Resistant Acinetobacter Isolates and Ampicillin-Resistant Non-Typhoidal Salmonella Species in Korea: A KONSAR Study Conducted in 2011
- Affiliations
-
- 1Department of Laboratory Medicine and Research Institute of Bacterial Resistance, Yonsei University College of Medicine, Seoul, Korea. whonetkor@yuhs.ac
- 2Department of Laboratory Medicine, Soonchunhyang University Bucheon Hospital, Bucheon, Korea.
- 3Samkwang Medical Laboratories, Seoul, Korea.
- 4Department of Laboratory Medicine, Wonkwang University Hospital, Iksan, Korea.
- 5Department of Laboratory Medicine, Ajou University Hospital, Suwon, Korea.
- 6Department of Laboratory Medicine, Dongguk University Gyeongju Hospital, Gyeongju, Korea.
- 7Department of Laboratory Medicine, Hanyang University College of Medicine, Seoul, Korea.
- KMID: 2284984
- DOI: http://doi.org/10.3947/ic.2014.46.2.84
Abstract
- BACKGROUND
Antimicrobial surveillance is important for providing an up-to-date understanding of the epidemiology of antimicrobial resistance and for creating a forum for rational drug development. In this study, we analyzed antimicrobial test data generated in 2011 by hospitals and commercial laboratories participating in the Korean Nationwide Surveillance of Antimicrobial Resistance program (KONSAR).
MATERIALS AND METHODS
Data on the results of susceptibility tests conducted in 32 hospitals and two commercial laboratories were analyzed. Data on isolates from patients admitted to an intensive care unit (ICU) and those admitted to other wards were compared. Intermediate susceptibility was not analyzed and duplicate isolates were excluded.
RESULTS
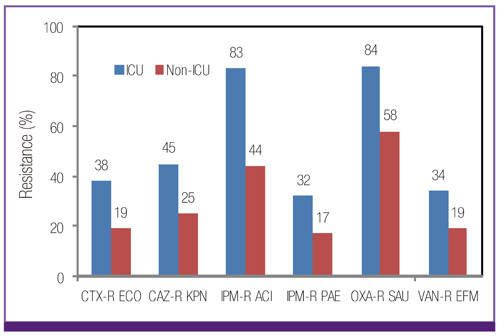
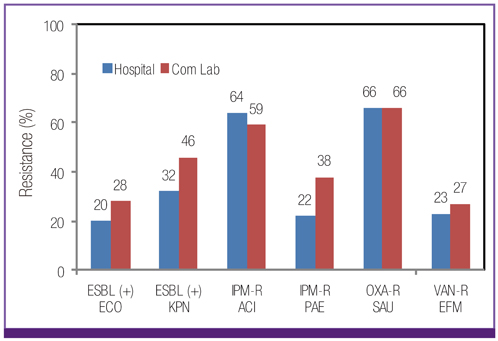
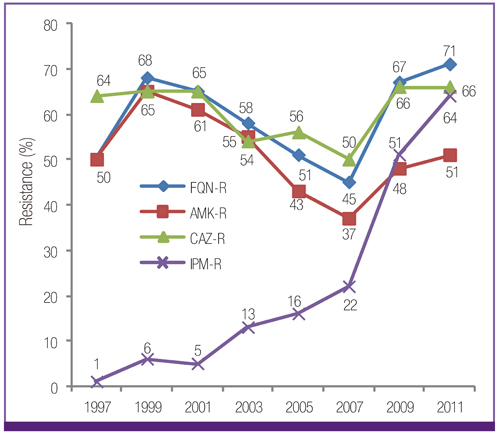
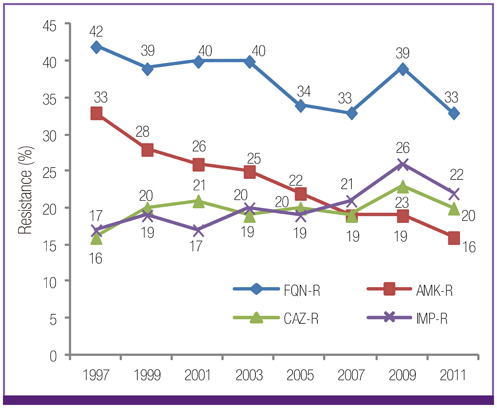
Escherichia coli was the most prevalent organism identified in both the hospital and commercial laboratories. Among the hospital isolates, methicillin-resistant Staphylococcus aureus (MRSA), penicillin G-non-susceptible Streptococcus pneumoniae, and ampicillin-resistant Enterococcus faecium remained as prevalent as they were in 2009. The proportion of vancomycin-resistant E. faecium (VR-EFM) slightly decreased from 29% in 2009 to 23% in 2011. Resistance rates of Klebsiella pneumoniae to ceftazidime, cefoxitin, fluoroquinolone, and amikacin were 24%, 14%, 27%, and 8%, respectively. Resistance rates of Pseudomonas aeruginosa to fluoroquinolone, ceftazidime, imipenem, and amikacin were 33%, 20%, 22%, and 16%, respectively, whereas those of Acinetobacter spp. resistance were 71%, 66%, 64, and 51%, respectively. The prevalence of oxyimino-cephalosporin-resistant E. coli and K. pneumoniae, carbapenem-resistant Acinetobacter spp. and P. aeruginosa, MRSA, and VR-EFM among ICU isolates was higher than those among non-ICU isolates. Extended-spectrum beta-lactamase-producing E. coli and K. pneumoniae, imipenem-resistant P. aeruginosa, and VR-EFM were more prevalent among isolates from commercial laboratories than those from hospitals. Resistance rates of K. pneumoniae to ceftazidime and amikacin decreased from 32% and 24% in 2005 to 24% and 8% in 2011, respectively. The resistance rate of P. aeruginosa to amikacin decreased from 22% in 2005 to 16% in 2011. The proportion of imipenem-resistant Acinetobacter spp. increased from 16% in 2005 to 64% in 2011.
CONCLUSIONS
The prevalence of MRSA, penicillin G-non-susceptible S. pneumoniae, and ampicillin-resistant E. faecium among clinical isolates tested in laboratories remained high. Multidrug resistance was more prevalent among isolates from ICUs. The prevalence of ceftazidime-resistant and amikacin-resistant K. pneumoniae and amikacin-resistant P. aeruginosa decreased after 2005, while the prevalence of imipenem-resistant Acinetobacter spp. increased.
Keyword
MeSH Terms
-
Acinetobacter*
Amikacin
Cefoxitin
Ceftazidime
Drug Resistance, Multiple
Enterococcus faecium
Epidemiology
Escherichia coli
Humans
Imipenem
Intensive Care Units
Klebsiella pneumoniae
Korea
Methicillin-Resistant Staphylococcus aureus
Penicillins
Pneumonia
Prevalence*
Pseudomonas aeruginosa
Salmonella*
Staphylococcus
Streptococcus pneumoniae
Amikacin
Cefoxitin
Ceftazidime
Imipenem
Penicillins
Figure
Cited by 7 articles
-
Increasing Carbapenem-Resistant Gram-Negative Bacilli and Decreasing Metallo-β-Lactamase Producers over Eight Years from Korea
Yangsoon Lee, Chang-Ki Kim, Hae-Sun Chung, Dongeun Yong, Seok Hoon Jeong, Kyungwon Lee, Yunsop Chong
Yonsei Med J. 2015;56(2):572-577. doi: 10.3349/ymj.2015.56.2.572.Improvement Plan for the Korean Nationwide Surveillance of Antimicrobial Resistance Program
Young Uh
Infect Chemother. 2014;46(2):141-142. doi: 10.3947/ic.2014.46.2.141.Characteristics of Metallo-β-Lactamase-Producing Pseudomonas aeruginosa in Korea
Jun Sung Hong, Jung Ok Kim, Hyukmin Lee, Il Kwon Bae, Seok Hoon Jeong, Kyungwon Lee
Infect Chemother. 2015;47(1):33-40. doi: 10.3947/ic.2015.47.1.33.Epidemiology and Characteristics of Metallo-β-Lactamase-Producing Pseudomonas aeruginosa
Duck Jin Hong, Il Kwon Bae, In-Ho Jang, Seok Hoon Jeong, Hyun-Kyung Kang, Kyungwon Lee
Infect Chemother. 2015;47(2):81-97. doi: 10.3947/ic.2015.47.2.81.Microorganisms Isolated from Urine Cultures and Their Antimicrobial Susceptibility Patterns at a Commercial Laboratory during 2018-2020
Byeonghak Kwak, Jungmi Hong, Hye Gyung Bae, Yoon Soo Park, Mi Kyeong Lee, Kyungwon Lee, Kyoung Ryul Lee
Korean J Healthc Assoc Infect Control Prev. 2022;27(1):51-58. doi: 10.14192/kjicp.2022.27.1.51.Current Status and Prospects of the National Antimicrobial Resistance Surveillance System, Kor-GLASS
Dokyun Kim, Min Hyuk Choi, Jun Sung Hong, Jong Hee Shin, Seok Hoon Jeong
Korean J Healthc Assoc Infect Control Prev. 2022;27(2):96-103. doi: 10.14192/kjicp.2022.27.2.96.Changing Genotypic Distribution, Antimicrobial Susceptibilities, and Risk Factors of Urinary Tract Infection Caused by Carbapenemase-Producing
Pseudomonas aeruginosa
Seri Jeong, Kibum Jeon, Nuri Lee, Min-Jeong Park, Wonkeun Song
Ann Lab Med. 2024;44(1):38-46. doi: 10.3343/alm.2024.44.1.38.
Reference
-
1. Chong Y, Lee K, Park YJ, Jeon DS, Lee MH, Kim MY, Chang CH, Kim EC, Lee NY, Kim HS, Kang ES, Cho HC, Paik IK, Lee HS, Jang SJ, Park AJ, Cha YJ, Kang SH, Lee MH, Song W, Shin JH. Korean nationwide surveillance of antimicrobial resistance of bacteria in 1997. Yonsei Med J. 1998; 39:569–577.
Article2. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; nineteenth informational supplement M100-S19. Wayne, PA: CLSI;2009.3. Clinical and Laboratory Standards Institute. CLSI document M100-S21. Performance standards for antimicrobial susceptibility testing; twenty-first informational supplement. Wayne, PA: CLSI;2011.4. O'Brien TF, Stelling JM. WHONET: removing obstacles to the full use of information about antimicrobial resistance. Diagn Microbiol Infect Dis. 1996; 25:162–168.5. Lee K, Lee MA, Lee CH, Lee J, Roh KH, Kim S, Kim JJ, Koh E, Yong D, Chong Y. KONSAR Group. Increase of ceftazidime-and fluoroquinolone-resistant Klebsiella pneumoniae and imipenem-resistant Acinetobacter spp. in Korea: analysis of KONSAR study data from 2005 and 2007. Yonsei Med J. 2010; 51:901–911.
Article6. Fridkin SK, Hill HA, Volkova NV, Edwards JR, Lawton RM, Gaynes RP, McGowan JE. Temporal changes in prevalence of antimicrobial resistance in 23 US hospitals. Emerg Infect Dis. 2002; 8:697–701.
Article7. Van Beneden CA, Lexau C, Baughman W, Barnes B, Bennett N, Cassidy PM, Pass M, Gelling L, Barrett NL, Zell ER, Whitney CG. Aggregated antibiograms and monitoring of drug-resistant Streptococcus pneumoniae. Emerg Infect Dis. 2003; 9:1089–1095.
Article8. Sahm DF, Marsilio MK, Piazza G. Antimicrobial resistance in key bloodstream bacterial isolates: electronic surveillance with the Surveillance Network Database--USA. Clin Infect Dis. 1999; 29:259–263.
Article9. Felmingham D, Grüneberg RN. The Alexander Project 1996-1997: latest susceptibility data from this international study of bacterial pathogens from community-acquired lower respiratory tract infections. J Antimicrob Chemother. 2000; 45:191–203.
Article10. Clinical and Laboratory Standards Institute. Analysis and presentation of cumulative antimicrobial susceptibility test data; approved guideline-Third Edition, M39-A3. Wayne, PA: CLSI;2008.11. Zhanel GG, DeCorby M, Laing N, Weshnoweski B, Vashisht R, Tailor F, Nichol KA, Wierzbowski A, Baudry PJ, Karlowsky JA, Lagacé-Wiens P, Walkty A, McCracken M, Mulvey MR, Johnson J, Hoban DJ. Canadian Antimicrobial Resistance Alliance (CARA). Antimicrobial-resistant pathogens in intensive care units in Canada: results of the Canadian National Intensive Care Unit (CAN-ICU) study, 2005-2006. Antimicrob Agents Chemother. 2008; 52:1430–1437.
Article12. Neuhauser MM, Weinstein RA, Rydman R, Danziger LH, Karam G, Quinn JP. Antibiotic resistance among gram-negative bacilli in US intensive care units: implications for fluoroquinolone use. JAMA. 2003; 289:885–888.
Article13. Rosenthal VD, Maki DG, Jamulitrat S, Medeiros EA, Todi SK, Gomez DY, Leblebicioglu H, Abu Khader I, Miranda Novales MG, Berba R, Ramírez Wong FM, Barkat A, Pino OR, Dueñas L, Mitrev Z, Bijie H, Gurskis V, Kanj SS, Mapp T, Hidalgo RF, Ben Jaballah N, Raka L, Gikas A, Ahmed A, Thu le TA, Guzmán Siritt ME. INICC Members. International Nosocomial Infection Control Consortium (INICC) report, data summary for 2003-2008, issued June 2009. Am J Infect Control. 2010; 38:95–104. e2
Article14. Lee K, Kim MN, Kim JS, Hong HL, Kang JO, Shin JH, Park YJ, Yong D, Jeong SH, Chong Y. KONSAR Group. Further increases in carbapenem-, amikacin-, and fluoroquinolone-resistant isolates of Acinetobacter spp. and P. aeruginosa in Korea: KONSAR study 2009. Yonsei Med J. 2011; 52:793–802.
Article15. Bae S, Lee J, Lee J, Kim E, Lee S, Yu J, Kang Y. Antimicrobial resistance in Haemophilus influenzae respiratory tract isolates in Korea: results of a nationwide acute respiratory infections surveillance. Antimicrob Agents Chemother. 2010; 54:65–71.
Article16. Lee K, Kim MN, Choi TY, Cho SE, Lee S, Whang DH, Yong D, Chong Y, Woodford N, Livermore DM. Wide dissemination of OXA-type carbapenemases in clinical Acinetobacter spp. isolates from South Korea. Int J Antimicrob Agents. 2009; 33:520–524.
Article17. Ko KS, Suh JY, Kwon KT, Jung SI, Park KH, Kang CI, Chung DR, Peck KR, Song JH. High rates of resistance to colistin and polymyxin B in subgroups of Acinetobacter baumannii isolates from Korea. J Antimicrob Chemother. 2007; 60:1163–1167.
Article18. Lee K, Yong D, Jeong SH, Chong Y. Multidrug-resistant Acinetobacter spp.: increasingly problematic nosocomial pathogens. Yonsei Med J. 2011; 52:879–891.
Article19. Cai Y, Chai D, Wang R, Liang B, Bai N. Colistin resistance of Acinetobacter baumannii: clinical reports, mechanisms and antimicrobial strategies. J Antimicrob Chemother. 2012; 67:1607–1615.
Article20. Sung H, Choi SJ, Yoo S, Kim MN. In vitro antimicrobial synergy against imipenem-resistant Acinetobacter baumannii. Korean J Lab Med. 2007; 27:111–117.
Article21. Poirel L, Potron A, Nordmann P. OXA-48-like carbapenemases: the phantom menace. J Antimicrob Chemother. 2012; 67:1597–1606.
Article22. Potron A, Rondinaud E, Poirel L, Belmonte O, Boyer S, Camiade S, Nordmann P. Genetic and biochemical characterisation of OXA-232, a carbapenem-hydrolysing class D β-lactamase from Enterobacteriaceae. Int J Antimicrob Agents. 2013; 41:325–329.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Non-typhoidal Salmonella Gastroenteritis in Childhood: Clinical Features and Antibiotics Resistance
- Antimicrobial Resistance and Clones of Acinetobacter Species and Pseudomonas aeruginosa
- Further Increases in Carbapenem-, Amikacin-, and Fluoroquinolone-Resistant Isolates of Acinetobacter spp. and P. aeruginosa in Korea: KONSAR Study 2009
- Carbapenem-Resistant Acinetobacter baumannii
- Carbapenem resistance in critically important human pathogens isolated from companion animals: a systematic literature review