J Adv Prosthodont.
2014 Oct;6(5):379-386. 10.4047/jap.2014.6.5.379.
Optimization of growth inducing factors for colony forming and attachment of bone marrow-derived mesenchymal stem cells regarding bioengineering application
- Affiliations
-
- 1Department of Prosthodontics & Dental Research Institute, Seoul National University Dental Hospital, School of Dentistry, Seoul National University, Seoul, Republic of Korea.
- 2Department of Prosthodontics, Asan Medical Center, College of Medicine, University of Ulsan, Seoul, Republic of Korea. ljhl11911@hanmail.net
- KMID: 2284738
- DOI: http://doi.org/10.4047/jap.2014.6.5.379
Abstract
- PURPOSE
These days, mesenchymal stem cells (MSCs) have received worldwide attention because of their potentiality in tissue engineering for implant dentistry. The purpose of this study was to evaluate various growth inducing factors in media for improvement of acquisition of bone marrow mesenchymal stem cells (BMMSCs) and colony forming unit-fibroblast (CFU-F).
MATERIALS AND METHODS
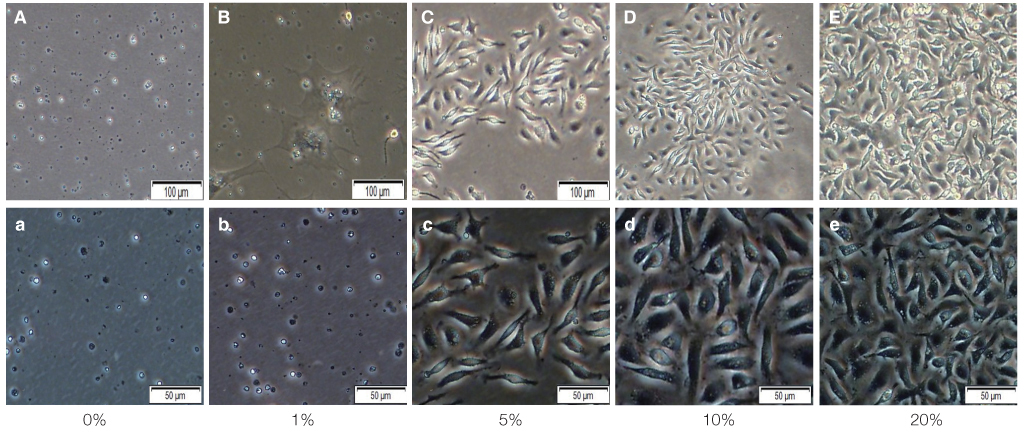
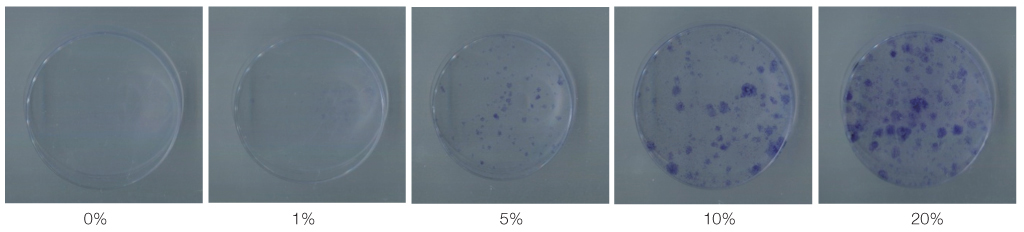
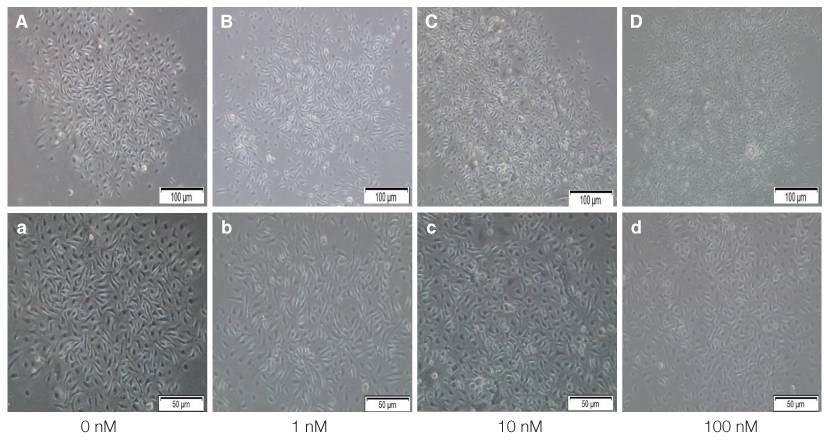
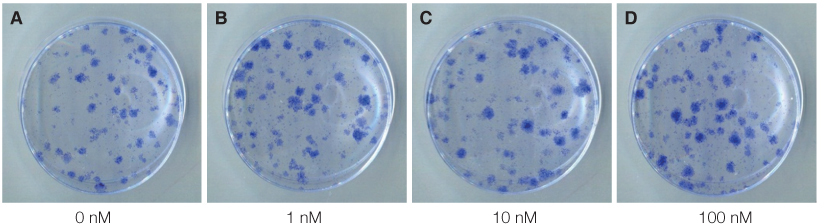
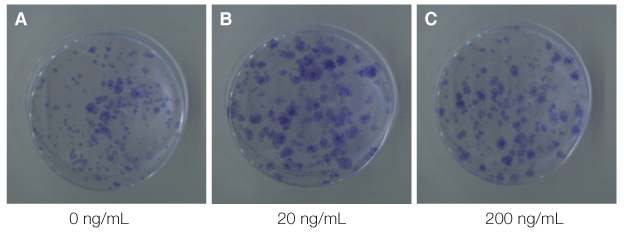
The mouse BMMSCs were freshly obtained from female C3H mouse femur and tibia. The cells seeded at the density of 106/dish in media supplemented with different density of fetal bovine serum (FBS), 1alpha, 25-dihydroxyvitamin (VD3) and recombinant human epidermal growth factor (rhEGF). After 14 days, CFU-F assay was conducted to analyze the cell attachment and proliferation, and moreover for VD3, the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay was additionally conducted.
RESULTS
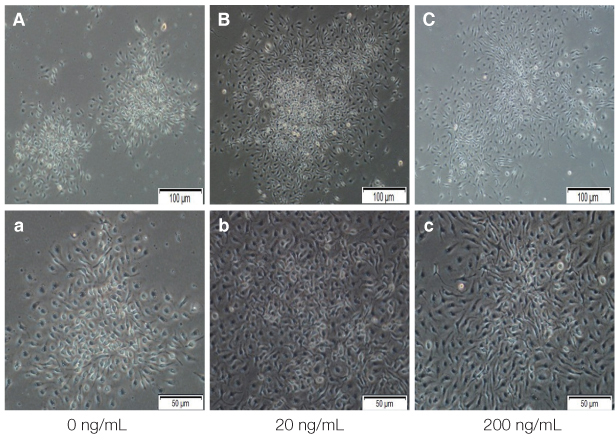
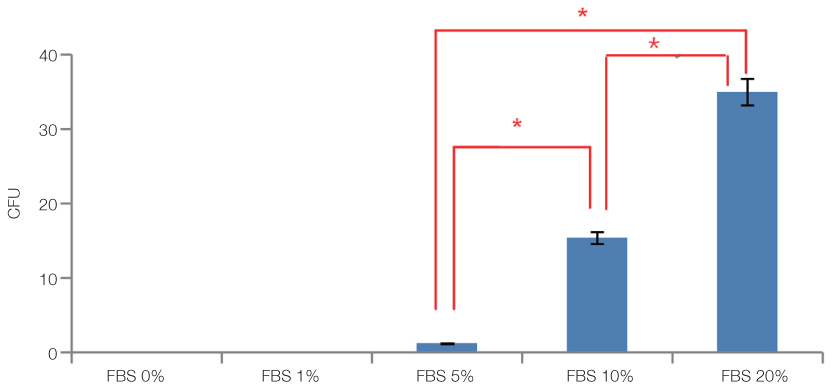
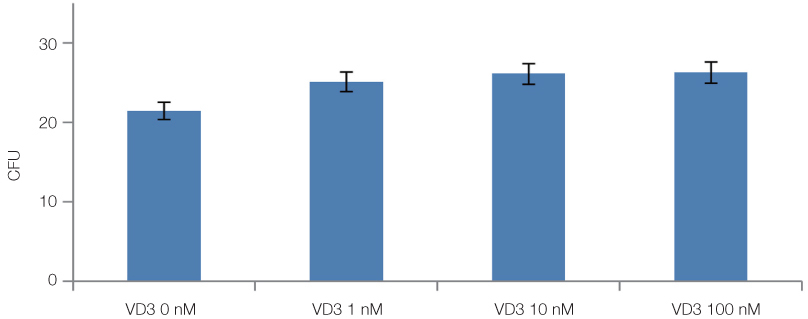
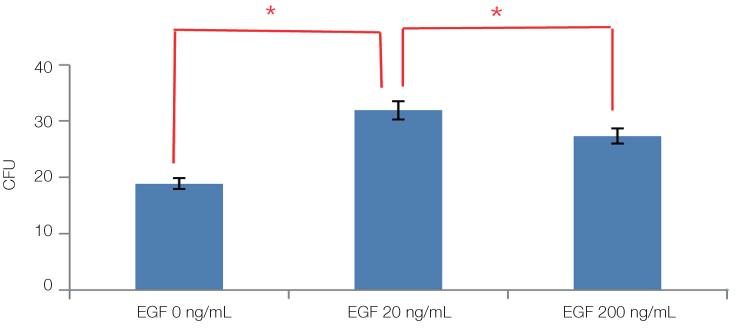
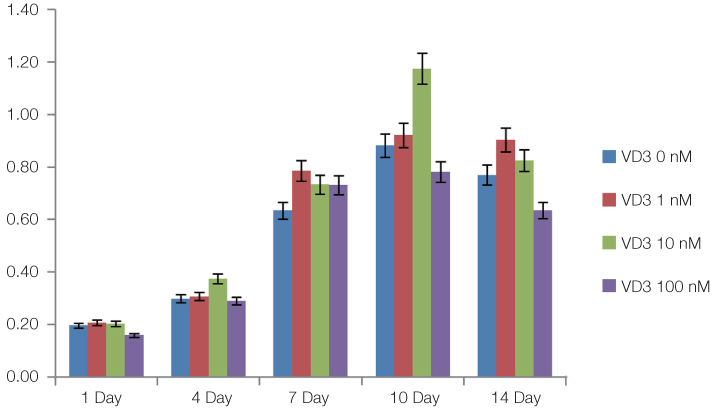
The cell proliferation was increased with the increase of FBS concentration (P<.05). The cell proliferation was highest at the density of 20 ng/mL rhEGF compared with 0 ng/mL and 200 ng/mL rhEGF (P<.05). For VD3, although the colony number was increased with the increase of its concentration, the difference was not statistically significant (P>.05).
CONCLUSION
FBS played the main role in cell attachment and growth, and the growth factor like rhEGF played the additional effect. However, VD3 did not have much efficacy compare with the other two factors. Improvement of the conditions could be adopted to acquire more functional MSCs to apply into bony defect around implants easily.
Keyword
MeSH Terms
Figure
Reference
-
1. Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004; 8:301–316.2. Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006; 24:1294–1301.3. Rodríguez-Lozano FJ, Insausti CL, Iniesta F, Blanquer M, Ramírez MD, Meseguer L, Meseguer-Henarejos AB, Marín N, Martínez S, Moraleda JM. Mesenchymal dental stem cells in regenerative dentistry. Med Oral Patol Oral Cir Bucal. 2012; 17:e1062–e1067.4. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284:143–147.5. Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002; 418:41–49.6. Nadri S, Soleimani M, Hosseni RH, Massumi M, Atashi A, Izadpanah R. An efficient method for isolation of murine bone marrow mesenchymal stem cells. Int J Dev Biol. 2007; 51:723–729.7. Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004; 103:1662–1668.8. Phinney DG, Kopen G, Isaacson RL, Prockop DJ. Plastic adherent stromal cells from the bone marrow of commonly used strains of inbred mice: variations in yield, growth, and differentiation. J Cell Biochem. 1999; 72:570–585.9. Hayman EG, Pierschbacher MD, Suzuki S, Ruoslahti E. Vitronectin--a major cell attachment-promoting protein in fetal bovine serum. Exp Cell Res. 1985; 160:245–258.10. Jochems CE, van der Valk JB, Stafleu FR, Baumans V. The use of fetal bovine serum: ethical or scientific problem? Altern Lab Anim. 2002; 30:219–227.11. Miyaura C, Abe E, Kuribayashi T, Tanaka H, Konno K, Nishii Y, Suda T. 1 alpha,25-Dihydroxyvitamin D3 induces differentiation of human myeloid leukemia cells. Biochem Biophys Res Commun. 1981; 102:937–943.12. Okuno H, Kishimoto KN, Hatori M, Itoi E. 1α,25-dihydroxyvitamin D3 enhances fast-myosin heavy chain expression in differentiated C2C12 myoblasts. Cell Biol Int. 2012; 36:441–447.13. Matsuda N, Morita N, Matsuda K, Watanabe M. Proliferation and differentiation of human osteoblastic cells associated with differential activation of MAP kinases in response to epidermal growth factor, hypoxia, and mechanical stress in vitro. Biochem Biophys Res Commun. 1998; 249:350–354.14. Soleimani M, Nadri S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc. 2009; 4:102–106.15. Zhu H, Guo ZK, Jiang XX, Li H, Wang XY, Yao HY, Zhang Y, Mao N. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat Protoc. 2010; 5:550–560.16. Dobson KR, Reading L, Haberey M, Marine X, Scutt A. Centrifugal isolation of bone marrow from bone: an improved method for the recovery and quantitation of bone marrow osteoprogenitor cells from rat tibiae and femurae. Calcif Tissue Int. 1999; 65:411–413.17. Kotton DN, Ma BY, Cardoso WV, Sanderson EA, Summer RS, Williams MC, Fine A. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development. 2001; 128:5181–5188.18. Baddoo M, Hill K, Wilkinson R, Gaupp D, Hughes C, Kopen GC, Phinney DG. Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J Cell Biochem. 2003; 89:1235–1249.19. Cholewa D, Stiehl T, Schellenberg A, Bokermann G, Joussen S, Koch C, Walenda T, Pallua N, Marciniak-Czochra A, Suschek CV, Wagner W. Expansion of adipose mesenchymal stromal cells is affected by human platelet lysate and plating density. Cell Transplant. 2011; 20:1409–1422.20. Tropel P, Noël D, Platet N, Legrand P, Benabid AL, Berger F. Isolation and characterisation of mesenchymal stem cells from adult mouse bone marrow. Exp Cell Res. 2004; 295:395–406.21. Li X, Zhang Y, Qi G. Evaluation of isolation methods and culture conditions for rat bone marrow mesenchymal stem cells. Cytotechnology. 2013; 65:323–334.22. Xue ML, Zhu H, Thakur A, Willcox M. 1 alpha,25-Dihydroxyvitamin D3 inhibits pro-inflammatory cytokine and chemokine expression in human corneal epithelial cells colonized with Pseudomonas aeruginosa. Immunol Cell Biol. 2002; 80:340–345.23. Finch JL, Dusso AS, Pavlopoulos T, Slatopolsky EA. Relative potencies of 1,25-(OH)(2)D(3) and 19-Nor-1,25-(OH)(2) D(2) on inducing differentiation and markers of bone formation in MG-63 cells. J Am Soc Nephrol. 2001; 12:1468–1474.24. Banerjee P, Chatterjee M. Antiproliferative role of vitamin D and its analogs-a brief overview. Mol Cell Biochem. 2003; 253:247–254.25. Kommagani R, Whitlatch A, Leonard MK, Kadakia MP. p73 is essential for vitamin D-mediated osteoblastic differentiation. Cell Death Differ. 2010; 17:398–407.26. Artaza JN, Sirad F, Ferrini MG, Norris KC. 1,25(OH)2vitamin D3 inhibits cell proliferation by promoting cell cycle arrest without inducing apoptosis and modifies cell morphology of mesenchymal multipotent cells. J Steroid Biochem Mol Biol. 2010; 119:73–83.27. Lee JH, Um S, Jang JH, Seo BM. Effects of VEGF and FGF-2 on proliferation and differentiation of human periodontal ligament stem cells. Cell Tissue Res. 2012; 348:475–484.28. Tamama K, Fan VH, Griffith LG, Blair HC, Wells A. Epidermal growth factor as a candidate for ex vivo expansion of bone marrow-derived mesenchymal stem cells. Stem Cells. 2006; 24:686–695.29. Lucarelli E, Beccheroni A, Donati D, Sangiorgi L, Cenacchi A, Del Vento AM, Meotti C, Bertoja AZ, Giardino R, Fornasari PM, Mercuri M, Picci P. Platelet-derived growth factors enhance proliferation of human stromal stem cells. Biomaterials. 2003; 24:3095–3100.30. You DH, Nam MJ. Effects of human epidermal growth factor gene-transfected mesenchymal stem cells on fibroblast migration and proliferation. Cell Prolif. 2013; 46:408–415.31. Bressan RB, Melo FR, Almeida PA, Bittencourt DA, Visoni S, Jeremias TS, Costa AP, Leal RB, Trentin AG. EGF-FGF2 stimulates the proliferation and improves the neuronal commitment of mouse epidermal neural crest stem cells (EPINCSCs). Exp Cell Res. 2014; 327:37–47.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparative characteristic study from bone marrow-derived mesenchymal stem cells
- Therapeutic Angiogenesis with Somatic Stem Cell Transplantation
- Characterization of Mesenchymal Stem Cells Derived from Rat Bone Marrow and Adipose Tissue: A Comparative Study
- Clinical Use of Mesenchymal Stem Cells in Bone Regeneration
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro