Diabetes Metab J.
2013 Feb;37(1):22-29. 10.4093/dmj.2013.37.1.22.
Brown Adipose Tissue as a Regulator of Energy Expenditure and Body Fat in Humans
- Affiliations
-
- 1Department of Nutrition, Tenshi College, Sapporo, Japan. saito@tenshi.ac.jp
- KMID: 2280738
- DOI: http://doi.org/10.4093/dmj.2013.37.1.22
Abstract
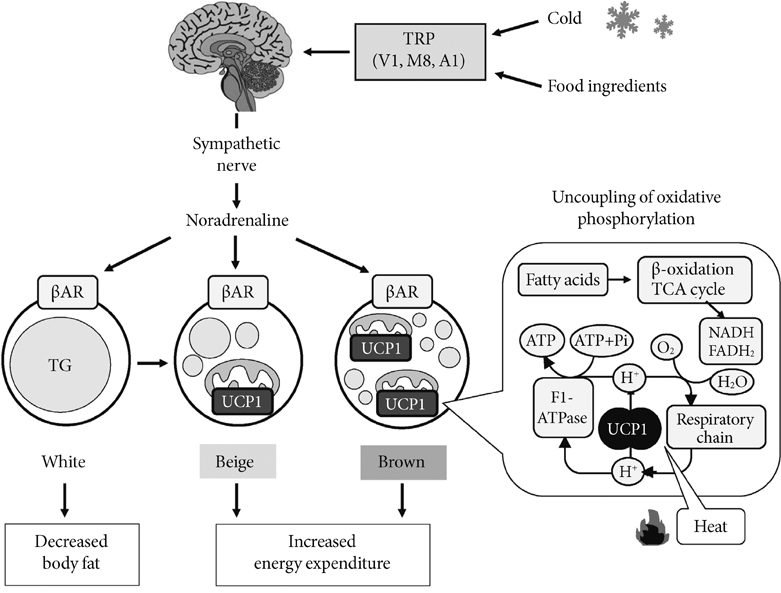
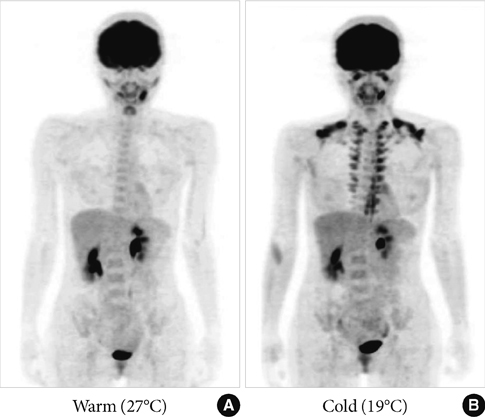
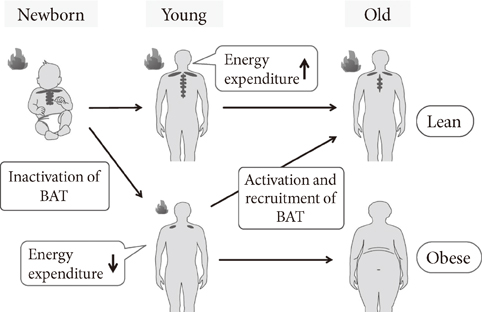
- Brown adipose tissue (BAT) is recognized as the major site of sympathetically activated nonshivering thermogenesis during cold exposure and after spontaneous hyperphagia, thereby controling whole-body energy expenditure and body fat. In adult humans, BAT has long been believed to be absent or negligible, but recent studies using fluorodeoxyglucose-positron emission tomography, in combination with computed tomography, demonstrated the existence of metabolically active BAT in healthy adult humans. Human BAT is activated by acute cold exposure, being positively correlated to cold-induced increases in energy expenditure. The metabolic activity of BAT differs among individuals, being lower in older and obese individuals. Thus, BAT is recognized as a regulator of whole-body energy expenditure and body fat in humans as in small rodents, and a hopeful target combating obesity and related disorders. In fact, there are some food ingredients such as capsaicin and capsinoids, which have potential to activate and recruit BAT via activity on the specific receptor, transient receptor potential channels, thereby increasing energy expenditure and decreasing body fat modestly and consistently.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Brown Fat as a Regulator of Systemic Metabolism beyond Thermogenesis
Okamatsu-Ogura Yuko, Masayuki Saito
Diabetes Metab J. 2021;45(6):840-852. doi: 10.4093/dmj.2020.0291.
Reference
-
1. Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004. 84:277–359.2. Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000. 404:652–660.3. Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007. 293:E444–E452.4. Ricquier D, Bouillaud F. The uncoupling protein homologues: UCP1, UCP2, UCP3, StUCP and AtUCP. Biochem J. 2000. 345(Pt 2):161–179.5. Shimizu Y, Nikami H, Saito M. Sympathetic activation of glucose utilization in brown adipose tissue in rats. J Biochem. 1991. 110:688–692.6. Inokuma K, Ogura-Okamatsu Y, Toda C, Kimura K, Yamashita H, Saito M. Uncoupling protein 1 is necessary for norepinephrine-induced glucose utilization in brown adipose tissue. Diabetes. 2005. 54:1385–1391.7. Himms-Hagen J. Does brown adipose tissue (BAT) have a role in the physiology or treatment of human obesity? Rev Endocr Metab Disord. 2001. 2:395–401.8. Cinti S. The role of brown adipose tissue in human obesity. Nutr Metab Cardiovasc Dis. 2006. 16:569–574.9. Heaton JM. The distribution of brown adipose tissue in the human. J Anat. 1972. 112(Pt 1):35–39.10. Cohade C, Osman M, Pannu HK, Wahl RL. Uptake in supraclavicular area fat ("USA-Fat"): description on 18F-FDG PET/CT. J Nucl Med. 2003. 44:170–176.11. Garcia CA, Van Nostrand D, Atkins F, Acio E, Butler C, Esposito G, Kulkarni K, Majd M. Reduction of brown fat 2-deoxy-2-[F-18]fluoro-D-glucose uptake by controlling environmental temperature prior to positron emission tomography scan. Mol Imaging Biol. 2006. 8:24–29.12. Parysow O, Mollerach AM, Jager V, Racioppi S, San Roman J, Gerbaudo VH. Low-dose oral propranolol could reduce brown adipose tissue F-18 FDG uptake in patients undergoing PET scans. Clin Nucl Med. 2007. 32:351–357.13. Soderlund V, Larsson SA, Jacobsson H. Reduction of FDG uptake in brown adipose tissue in clinical patients by a single dose of propranolol. Eur J Nucl Med Mol Imaging. 2007. 34:1018–1022.14. Saito M, Okamatsu-Ogura Y, Tsujisaki M, Kaji T, Nakada K. Human brown adipose tissue evaluated by FDG-PET: activation by cold exposure. Int J Obes (Lond). 2007. 31:Suppl 1. S32.15. Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, Kawai Y, Tsujisaki M. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009. 58:1526–1531.16. van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009. 360:1500–1508.17. Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009. 360:1518–1525.18. Yoneshiro T, Aita S, Matsushita M, Okamatsu-Ogura Y, Kameya T, Kawai Y, Miyagawa M, Tsujisaki M, Saito M. Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obesity (Silver Spring). 2011. 19:1755–1760.19. Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009. 360:1509–1517.20. Au-Yong IT, Thorn N, Ganatra R, Perkins AC, Symonds ME. Brown adipose tissue and seasonal variation in humans. Diabetes. 2009. 58:2583–2587.21. Pfannenberg C, Werner MK, Ripkens S, Stef I, Deckert A, Schmadl M, Reimold M, Haring HU, Claussen CD, Stefan N. Impact of age on the relationships of brown adipose tissue with sex and adiposity in humans. Diabetes. 2010. 59:1789–1793.22. Ouellet V, Routhier-Labadie A, Bellemare W, Lakhal-Chaieb L, Turcotte E, Carpentier AC, Richard D. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J Clin Endocrinol Metab. 2011. 96:192–199.23. Nedergaard J, Bengtsson T, Cannon B. Three years with adult human brown adipose tissue. Ann N Y Acad Sci. 2010. 1212:E20–E36.24. Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature. 1997. 387:90–94.25. Yoneshiro T, Aita S, Matsushita M, Kameya T, Nakada K, Kawai Y, Saito M. Brown adipose tissue, whole-body energy expenditure, and thermogenesis in healthy adult men. Obesity (Silver Spring). 2011. 19:13–16.26. Orava J, Nuutila P, Lidell ME, Oikonen V, Noponen T, Viljanen T, Scheinin M, Taittonen M, Niemi T, Enerback S, Virtanen KA. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab. 2011. 14:272–279.27. Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature. 1979. 281:31–35.28. Kozak LP. Brown fat and the myth of diet-induced thermogenesis. Cell Metab. 2010. 11:263–267.29. Del Mar Gonzalez-Barroso M, Ricquier D, Cassard-Doulcier AM. The human uncoupling protein-1 gene (UCP1): present status and perspectives in obesity research. Obes Rev. 2000. 1:61–72.30. Rose G, Crocco P, D'Aquila P, Montesanto A, Bellizzi D, Passarino G. Two variants located in the upstream enhancer region of human UCP1 gene affect gene expression and are correlated with human longevity. Exp Gerontol. 2011. 46:897–904.31. Esterbauer H, Oberkofler H, Liu YM, Breban D, Hell E, Krempler F, Patsch W. Uncoupling protein-1 mRNA expression in obese human subjects: the role of sequence variations at the uncoupling protein-1 gene locus. J Lipid Res. 1998. 39:834–844.32. Yoneshiro T, Ogawa T, Okamoto N, Matsushita M, Aita S, Kameya T, Kawai Y, Iwanaga T, Saito M. Impact of UCP1 and beta3AR gene polymorphisms on age-related changes in brown adipose tissue and adiposity in humans. Int J Obes (Lond). Epub 2012 Oct 2. DOI: http://dx.doi.org/10.1038/ijo.2012.161.33. Nagai N, Sakane N, Fujishita A, Fujiwara R, Kimura T, Kotani K, Moritani T. The -3826 A-->G variant of the uncoupling protein-1 gene diminishes thermogenesis during acute cold exposure in healthy children. Obes Res Clin Pract. 2007. 1:99–107.34. Nagai N, Sakane N, Ueno LM, Hamada T, Moritani T. The -3826 A-->G variant of the uncoupling protein-1 gene diminishes postprandial thermogenesis after a high fat meal in healthy boys. J Clin Endocrinol Metab. 2003. 88:5661–5667.35. Kontani Y, Wang Y, Kimura K, Inokuma KI, Saito M, Suzuki-Miura T, Wang Z, Sato Y, Mori N, Yamashita H. UCP1 deficiency increases susceptibility to diet-induced obesity with age. Aging Cell. 2005. 4:147–155.36. Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009. 9:203–209.37. St-Onge MP, Gallagher D. Body composition changes with aging: the cause or the result of alterations in metabolic rate and macronutrient oxidation? Nutrition. 2010. 26:152–155.38. Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, Penicaud L, Casteilla L. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci. 1992. 103(Pt 4):931–942.39. Guerra C, Koza RA, Yamashita H, Walsh K, Kozak LP. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J Clin Invest. 1998. 102:412–420.40. Inokuma K, Okamatsu-Ogura Y, Omachi A, Matsushita Y, Kimura K, Yamashita H, Saito M. Indispensable role of mitochondrial UCP1 for antiobesity effect of beta3-adrenergic stimulation. Am J Physiol Endocrinol Metab. 2006. 290:E1014–E1021.41. Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem. 2010. 285:7153–7164.42. Kajimura S, Seale P, Spiegelman BM. Transcriptional control of brown fat development. Cell Metab. 2010. 11:257–262.43. Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S, Spiegelman BM. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011. 121:96–105.44. Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerback S, Schrauwen P, Spiegelman BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012. 150:366–376.45. Lee P, Swarbrick MM, Zhao JT, Ho KK. Inducible brown adipogenesis of supraclavicular fat in adult humans. Endocrinology. 2011. 152:3597–3602.46. Caterina MJ. Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am J Physiol Regul Integr Comp Physiol. 2007. 292:R64–R76.47. Whiting S, Derbyshire E, Tiwari BK. Capsaicinoids and capsinoids A potential role for weight management? A systematic review of the evidence. Appetite. 2012. 59:341–348.48. Yoneshiro T, Aita S, Kawai Y, Iwanaga T, Saito M. Nonpungent capsaicin analogs (capsinoids) increase energy expenditure through the activation of brown adipose tissue in humans. Am J Clin Nutr. 2012. 95:845–850.49. Saito M, Yoneshiro T. Capsinoids and related food ingredients activating brown fat thermogenesis and reducing body fat in humans. Curr Opin Lipidol. 2013. 24:71–77.50. Vallerand AL, Perusse F, Bukowiecki LJ. Cold exposure potentiates the effect of insulin on in vivo glucose uptake. Am J Physiol. 1987. 253(2 Pt 1):E179–E186.51. Vallerand AL, Perusse F, Bukowiecki LJ. Stimulatory effects of cold exposure and cold acclimation on glucose uptake in rat peripheral tissues. Am J Physiol. 1990. 259(5 Pt 2):R1043–R1049.52. Shimizu Y, Nikami H, Tsukazaki K, Machado UF, Yano H, Seino Y, Saito M. Increased expression of glucose transporter GLUT-4 in brown adipose tissue of fasted rats after cold exposure. Am J Physiol. 1993. 264(6 Pt 1):E890–E895.53. Gasparetti AL, de Souza CT, Pereira-da-Silva M, Oliveira RL, Saad MJ, Carneiro EM, Velloso LA. Cold exposure induces tissue-specific modulation of the insulin-signalling pathway in Rattus norvegicus. J Physiol. 2003. 552(Pt 1):149–162.54. Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, Eychmuller A, Gordts PL, Rinninger F, Bruegelmann K, Freund B, Nielsen P, Merkel M, Heeren J. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 2011. 17:200–205.55. Nishio M, Yoneshiro T, Nakahara M, Suzuki S, Saeki K, Hasegawa M, Kawai Y, Akutsu H, Umezawa A, Yasuda K, Tobe K, Yuo A, Kubota K, Saito M. Production of functional classical brown adipocytes from human pluripotent stem cells using specific hemopoietin cocktail without gene transfer. Cell Metab. 2012. 16:394–406.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Brown Adipose Tissue as a Therapeutic Target for Obesity: From Mice to Humans
- Thermogenic Brown Fat in Humans: Implications in Energy Homeostasis, Obesity and Metabolic Disorders
- Brown Fat and Browning for the Treatment of Obesity and Related Metabolic Disorders
- Brown Fat as a Regulator of Systemic Metabolism beyond Thermogenesis
- HOXC10 suppresses browning of white adipose tissues