Chonnam Med J.
2009 Dec;45(3):154-160. 10.4068/cmj.2009.45.3.154.
Clinical Correlation of CD4+CD25+ Regulatory T Cells in Early Immune Reconstitution after Myeloablative Allogeneic Stem Cell Transplantation
- Affiliations
-
- 1Hematology-Oncology, Chonnam National University Hwasun Hospital, Hwasun, Jeonnam, Korea.
- 2Research Institute of Medical Sciences, Chonnam National University, Gwangju, Korea. drydh1685@hotmail.com
- KMID: 2274889
- DOI: http://doi.org/10.4068/cmj.2009.45.3.154
Abstract
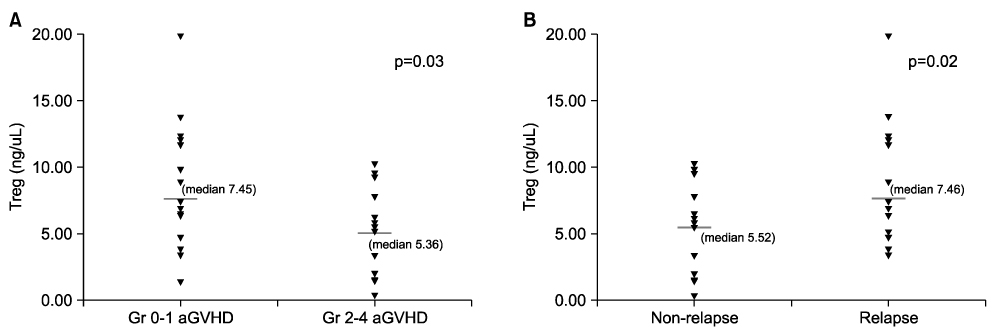
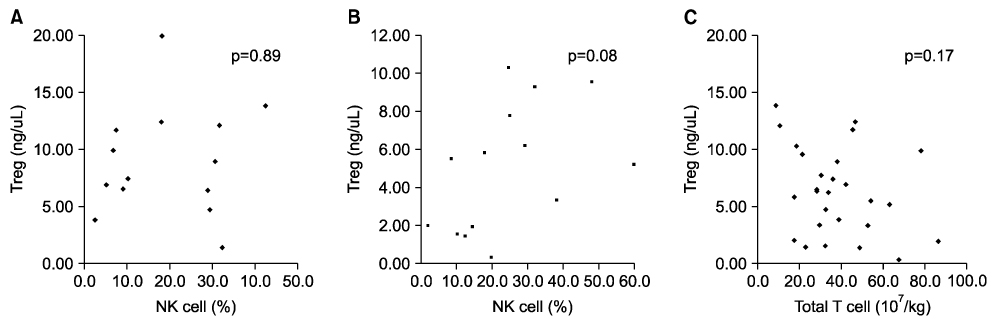
- We investigated whether CD4+CD25+ regulatory T cells (Tregs) not only reduce the incidence of acute graft-versus-host disease (aGVHD) but also inhibit the expression of NK cells during early immune reconstitution after allogeneic myeloablative hematopoietic stem cell transplantation (HSCT). In addition, we evaluated whether Tregs were associated with disease relapse. Twenty-nine patients underwent non-T cell-depleted allogeneic HSCT. Peripheral blood mononuclear cells (PBMCs) were separated at 3 weeks after HSCT. Fourteen patients developed grade 2-4 aGVHD and 13 patients relapsed. Patients with grade 2-4 aGVHD (median, 5.36 ng/microliter) had significantly lower levels of FOXP3 gene expression than did those with grade 0-1 aGVHD (median, 7.45 ng/microliter)ever, the level of FOXP3 . Howgene expression in patients with relapse (median, 7.46 ng/microliter) was significantly higher than in those (median, 5.52 ng/microliter) without relapse (P=0.02). However, we did not find an inverse correlation between the expression of NK cells and Tregs. The level of FOXP3 expression in CD4+CD25+ Tregs was related with the incidence of aGVHD and was associated with the risk of disease relapse. However, NK cells showed no correlation with the expression of Tregs during early immune reconstitution after myeloablative HSCT.
Keyword
MeSH Terms
Figure
Reference
-
1. Shlomchik WD, Couzens MS, Tang CB, McNiff J, Robert ME, Liu J, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999. 285:412–415.
Article2. Matte CC, Liu J, Cormier J, Anderson BE, Athanasiadis I, Jain D, et al. Donor APCs are required for maximal GVHD but not for GVL. Nat Med. 2004. 10:987–992.
Article3. Blazar BR, Taylor PA, Linsley PS, Vallera DA. In vivo blockade of CD28/CTLA4: B7/BB1 interaction with CTLA4-Ig reduces lethal murine graft-versus-host disease across the major histocompatibility complex barrier in mice. Blood. 1994. 83:3815–3825.
Article4. Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002. 99:3493–3499.
Article5. Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002. 196:389–399.
Article6. Hoffmann P, Edinger M. CD4+CD25+ regulatory T cells and graft-versus-host disease. Semin Hematol. 2006. 43:62–69.
Article7. Oluwole SF, Oluwole OO, DePaz HA, Adeyeri AO, Witkowski P, Hardy MA. CD4+CD25+ regulatory T cells mediate acquired transplant tolerance. Transpl Immunol. 2003. 11:287–293.
Article8. Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003. 299:1057–1061.
Article9. Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003. 4:337–342.
Article10. Yagi H, Nomura T, Nakamura K, Yamazaki S, Kitawaki T, Hori S, et al. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol. 2004. 16:1643–1656.
Article11. Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999. 163:5211–5218.12. Romagnani C, Della Chiesa M, Kohler S, Moewes B, Radbruch A, Moretta L, et al. Activation of human NK cells by plasmacytoid dendritic cells and its modulation by CD4 T helper cells and CD+CD25hi T regulatory cells. Eur J Immunol. 2005. 35:2452–2458.
Article13. Dieckmann D, Bruett CH, Ploettner H, Lutz MB, Schuler G. Human CD4+CD25+ regulatory, contact-dependent T cells induce interleukin 10-producing, contact-independent type 1-like regulatory T cells [corrected]. J Exp Med. 2002. 196:247–253.
Article14. Suri-Payer E, Amar AZ, Thornton AM, Shevach EM. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol. 1998. 160:1212–1218.15. Piccirillo CA, Shevach EM. Cutting edge: control of CD8+ T cell activation by CD4CD25 immunoregulatory cells. J Immunol. 2001. 167:1137–1140.
Article16. Trenado A, Charlotte F, Fisson S, Yagello M, Klatzmann D, Salomon BL, et al. Recipient-type specific CD4+CD25+ regulatory T cells favor immune reconstitution and control graft-versus-host disease while maintaining graft-versus-leukemia. J Clin Invest. 2003. 112:1688–1696.
Article17. Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003. 9:1144–1150.
Article18. Barrett J. Improving outcome of allogeneic stem cell transplantation by immunomodulation of the early post-transplant environment. Curr Opin Immunol. 2006. 18:592–598.
Article19. Wils EJ, Cornelissen JJ. Thymopoiesis following allogeneic stem cell transplantation: new possibilities for improvement. Blood Rev. 2005. 19:89–98.
Article20. Rezvani K, Brenchley JM, Price DA, Kilical Y, Gostick E, Sewell AK, et al. T-cell responses directed against multiple HLA-A*0201-restricted epitopes derived from Wilms' tumor 1 protein in patients with leukemia and healthy donors: identification, quantification, and characterization. Clin Cancer Res. 2005. 11:8799–8807.
Article21. Barrett J, Rezvani K. Neutrophil granule proteins as targets of leukemia-specific immune responses. Curr Opin Hematol. 2006. 13:15–20.
Article22. Michalek J, Collins RH, Durrani HP, Vaclavkova P, Ruff LE, Douek DC, et al. Definitive separation of graft-versus-leukemia- and graft-versus-host-specific CD4+ T cells by virtue of their receptor beta loci sequences. Proc Natl Acad Sci USA. 2003. 100:1180–1184.23. Wei WZ, Morris GP, Kong YC. Anti-tumor immunity and autoimmunity: a balancing act of regulatory T cells. Cancer Immunol Immunother. 2004. 53:73–78.
Article24. Grauer OM, Nierkens S, Bennink E, Toonen LW, Boon L, Wesseling P, et al. CD4+FoxP3+ regulatory T cells gradually accumulate in gliomas during tumor growth and efficiently suppress antiglioma immune responses in vivo. Int J Cancer. 2007. 121:95–105.
Article25. Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003. 9:606–612.26. Rezvani K, Mielke S, Ahmadzadeh M, Kilical Y, Savani BN, Zeilah J, et al. High donor FOXP3-positive regulatory T-cell (Treg) content is associated with a low risk of GVHD following HLA-matched allogeneic SCT. Blood. 2006. 108:1291–1297.
Article27. Schneider M, Munder M, Karakhanova S, Ho AD, Goerner M. The initial phase of graft-versus-host disease is associated with a decrease of CD4+CD25+ regulatory T cells in the peripheral blood of patients after allogeneic stem cell transplantation. Clin Lab Haematol. 2006. 28:382–390.
Article28. Barge RM, Starrenburg CW, Falkenburg JH, Fibbe WE, Marijt EW, Willemze R. Long-term follow-up of myeloablative allogeneic stem cell transplantation using Campath "in the bag" as T-cell depletion: the Leiden experience. Bone Marrow Transplant. 2006. 37:1129–1134.
Article29. Jiang Z, Adams GB, Hanash AM, Scadden DT, Levy RB. The contribution of cytotoxic and noncytotoxic function by donor T-cells that support engraftment after allogeneic bone marrow transplantation. Biol Blood Marrow Transplant. 2002. 8:588–596.
Article30. Kim DH, Won DI, Lee NY, Sohn SK, Suh JS, Lee KB. Non-CD34+ cells, especially CD8+ cytotoxic T cells and CD56+ natural killer cells, rather than CD34 cells, predict early engraftment and better transplantation outcomes in patients with hematologic malignancies after allogeneic peripheral stem cell transplantation. Biol Blood Marrow Transplant. 2006. 12:719–728.
Article31. Urbano-Ispizua A, Rozman C, Pimentel P, Solano C, de la Rubia J, Brunet S, et al. The number of donor CD3(+) cells is the most important factor for graft failure after allogeneic transplantation of CD34(+) selected cells from peripheral blood from HLA-identical siblings. Blood. 2001. 97:383–387.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Regulatory T Cells and Allogeneic Transplantation
- Distribution of CD4+CD25+ T cells and graft-versus-host disease in human hematopoietic stem cell transplantation
- Peripheral Generation of CD4+ CD25+ Foxp3+ Regulatory T Cells
- Inhibition of Graft Versus Host Disease Using CD4+ CD25+ T Cells Induced with Interleukin-2 in Mismatched Allogeneic Murine Hematopoietic Stem Cell Transplantation
- A Study on the Number of Circulating CD4+CD25+Foxp3+ Regulatory T Cells and CD4+CD25-Foxp3+ T Cells in Psoriasis