Cancer Res Treat.
2006 Apr;38(2):92-98.
Overexpression of alpha-protease Inhibitor and Galectin-1 in Radiation-induced Early Phase of Pulmonary Fibrosis
- Affiliations
-
- 1Laboratory of Radiation Immunology, Korea Institute of Radiological and Medical Science, KAERI, Seoul, Korea.
- 2Laboratory of Biopharmaceutical Processes, Graduate School of Life Sciences and Biotechnology, Korea University, Seoul, Korea.
Abstract
-
PURPOSE: Radiation-induced pulmonary fibrosis (RIF) is a significant complication of radiotherapy for lung cancer. Despite the large number of studies, the molecular mechanisms of RIF are poorly understood. Therefore, the complex protein expression pattern in RIF was characterized by identifying the proteins with an altered expression level after thorax irradiation using two-dimensional electrophoresis (2-DE) and mass spectrometry.
MATERIALS AND METHODS
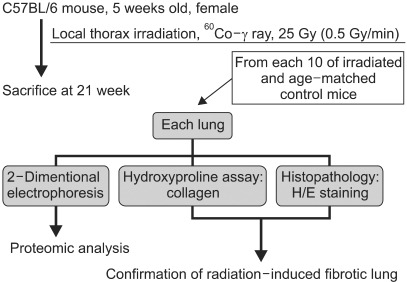
A mouse model of RIF was used to examine the alteration of the lung proteome because of availability of murine data related to human cases and the abundance of murine fibrotic lung samples. A mouse model of RIF was induced in radiosensitive C57BL/6 mice. Twenty-one weeks after 25 Gy irradiation, hematoxylin-eosin staining and hydroxyproline assay confirmed the early-phase pulmonary fibrosis.
RESULTS
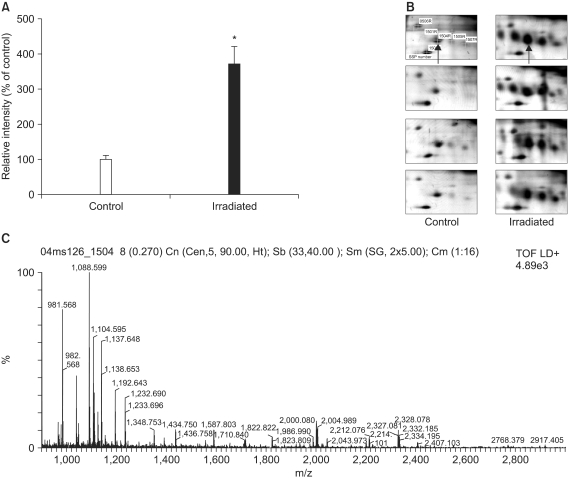
Lung samples from the irradiated and age-matched control mice were used to generate 16 high quality 2-DE gels containing approximately 1,000 spots. Of the 31 significantly up- or down-regulated protein spots, 17 were identified by MALDI-TOF/MS.
CONCLUSIONS
Two important upregulated proteins were found, the alpha-protease inhibitor and galectin-1, which might be used as potential markers for the early phase of RIF.
MeSH Terms
Figure
Reference
-
1. Morgan GW, Breit SN. Radiation and the lung: A reevaluation of the mechanisms mediating pulmonary injury. Int J Radiat Oncol Biol Phys. 1995; 31:361–369. PMID: 7836090.
Article2. Abid SH, Malhotra V, Perry MC. Radiation-induced and chemotherapy-induced pulmonary injury. Curr Opin Oncol. 2001; 13:242–248. PMID: 11429481.
Article3. Kaminski N, Allard JD, Pittet JF, Zuo F, Griffiths MJ, Morris D, et al. Global analysis of gene expression in pulmonary fibrosis reveals distinct programs regulating lung inflammation and fibrosis. Proc Natl Acad Sci USA. 2000; 97:1778–1783. PMID: 10677534.
Article4. Kelley J, Chrin L, Shull S, Rowe DW, Cutroneo KR. Bleomycin selectively elevates mRNA levels for procollagen and fibronectin following acute lung injury. Biochem Biophys Res Commun. 1985; 131:836–843. PMID: 2413849.
Article5. Abdollahi A, Li M, Ping G, Plathow C, Domhan S, Kiessling F, et al. Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. J Exp Med. 2005; 201:925–935. PMID: 15781583.
Article6. Finkelstein JN, Johnston CJ, Baggs R, Rubin P. Early alterations in extracellular matrix and transforming growth factor beta gene expression in mouse lung indicative of late radiation fibrosis. Int J Radiat Oncol Biol Phys. 1994; 28:621–631. PMID: 8113105.7. Delanian S, Lefaix J. The radiation-induced fibrotrophic process: therapeutic perspective via the antioxidant pathway. Radiother Oncol. 2004; 74:119–131. PMID: 15542158.8. Martin M, Lefaix J, Delanian S. TGF-beta1 and radiation fibrosis: a master switch and a specific therapeutic target? Int J Radiat Oncol Biol Phys. 2000; 47:277–290. PMID: 10802350.9. Rube CE, Uthe D, Schmid KW, Richter KD, Wessel J, Schuck A, et al. Dose-dependent induction of transforming growth factor β (TGF-β) in the lung tissue of fibrosis prone mice after thoracic irradiation. Int J Radiat Oncol Biol Phys. 2000; 47:1033–1042. PMID: 10863076.10. Reddy GK, Enwemeka CS. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem. 1996; 29:225–229. PMID: 8740508.11. Joo W, Lee D, Kim CW. Development of an effective sample preparation method for the proteome analysis of body fluids using 2-D gel electrophoresis. Biosci Biotechnol Biochem. 2003; 67:1574–1577. PMID: 12913303.
Article12. Joo W, Sul D, Lee DY, Lee E, Kim CW. Proteomic analysis of plasma proteins of workers exposed to benzene. Mutat Res. 2004; 558:35–44. PMID: 15036117.
Article13. Gharahdaghi F, Weinberg CR, Meagher DA, Imai BS, Mische SM. Mass spectrometric identification of proteins from silver-stained polyacrylamide gel: a method for the removal of silver ions to enhance sensitivity. Electrophoresis. 1999; 20:601–605. PMID: 10217175.
Article14. King TE. Update in pulmonary medicine. Ann Intern Med. 1998; 129:806–812. PMID: 9841587.
Article15. Franko AJ, Sharplin J, Ghahary A, Barcellos-Hoff MH. Immunohistochemical localization of transforming growth factor beta and tumor necrosis factor alpha in the lungs of fibrosisprone and non-fibrosing mice during the latent period and early phase after irradiation. Radiat Res. 1997; 147:245–256. PMID: 9008217.16. Johnston CJ, Williams JP, Okunieff P, Finkelstein JN. Radaition-induced pulmonary fibrosis: examination of chemokine and chemokine receptors families. Radiat Res. 2002; 157:256–265. PMID: 11839087.17. Kasper M, Hughes RC. Immunocytochemical evidence for a modulation of galectin-3 (Mac-2), a carbohydrate binding protein, in pulmonary fibrosis. J Pathol. 1996; 179:309–316. PMID: 8774488.18. Sanford GL, Harris-Hooker S. Stimulation of vascular cell proliferation by beta-galactoside specific lectins. FASEB J. 1990; 4:2912–2918. PMID: 2379767.19. Maeda N, Kawada N, Seki S, Arakawa T, Ikeda K, Iwao H, et al. Stimulation of proliferation of rat hepatic stellate cells by galectin-1 and galectin-3 through different intracellular signaling pathways. J Biol Chem. 2003; 278:18938–18944. PMID: 12646584.
Article20. Rabinovich GA, Daly G, Dreja H, Tailor H, Riera CM, Hirabayashi J, et al. Recombinant galectin-1 and its genetic delivery suppress collagen-induced arthritis via T cell apoptosis. J Exp Med. 1999; 190:385–398. PMID: 10430627.
Article21. Rabinovich GA, Sotomayor CE, Riera CM, Bianco I, Correa SG. Evidence of a role for galectin-1 in acute inflammation. Eur J Immunol. 2000; 30:1331–1339. PMID: 10820379.
Article22. Johnston CJ, Williams JP, Elder A, Hernady E, Finkelstein JN. Inflammatory cell recruitment following thoracic irradiation. Exp Lung Res. 2004; 30:369–382. PMID: 15204829.
Article23. Taooka Y, Maeda A, Hiyama K, Ishioka S, Yamakido M. Effects of neutrophil elastase inhibitor on bleomycin-induced pulmonary fibrosis in mice. Am J Respir Crit Care Med. 1997; 156:260–265. PMID: 9230758.
Article24. Yamanouchi H, Fujita J, Hojo S, Yoshinouchi T, Kamei T, Yamadori I, et al. Neutrophil elastase: α1-proteinase inhibitor complex in serum and bronchoalveolar lavage fluid in patients with pulmonary fibrosis. Eur Respir J. 1998; 11:120–125. PMID: 9543280.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Amelioration of Bleomycin-induced Pulmonary Fibrosis of Rats by an Aldose Reductase Inhibitor, Epalrestat
- Reducing Effect of Angiotensin-1 Converting Enzyme Inhibitor (Captopril) in Fibrosis of Radiation Induced Lung Injury
- Mesenchymal Stem Cells Ameliorate Fibrosis by Enhancing Autophagy via Inhibiting Galectin-3/Akt/mTOR Pathway and by Alleviating the EMT via Inhibiting Galectin-3/Akt/GSK3β/Snail Pathway in NRK-52E Fibrosis
- The Radioprotective Effect and Mechanism of Captopril on Radiation Induced Lung Damage in Rat
- Histomorphologic Change of Radiation Pneumonitis in Rat Lungs : Captopril Reduces Rat Lung Injury Induced by Irradiation