Cancer Res Treat.
2010 Sep;42(3):151-156.
Up-regulation of RhoGDI2 in Human Breast Cancer and Its Prognostic Implications
- Affiliations
-
- 1Department of Surgery, Seoul National University Hospital, Seoul, Korea.
- 2Department of Surgery, Gyeongsang Institute of Health Science, Gyeongsang National University School of Medicine, Jinju, Korea. drjej@gnu.ac.kr
- 3Department of Pathology, Gyeongsang Institute of Health Science, Gyeongsang National University School of Medicine, Jinju, Korea.
Abstract
- PURPOSE
Recent research has identified many genes and proteins that play specific roles in the process of systemic metastasis in various types of cancer. Rho GDP dissociation inhibitor 2 (RhoGDI2) has been shown to inhibit metastasis in human bladder cancer, but its role in breast cancer is controversial.
MATERIALS AND METHODS
We examined the regulation and clinical significance of RhoGDI2 in Korean breast cancer patients by using proteomic approaches.
RESULTS
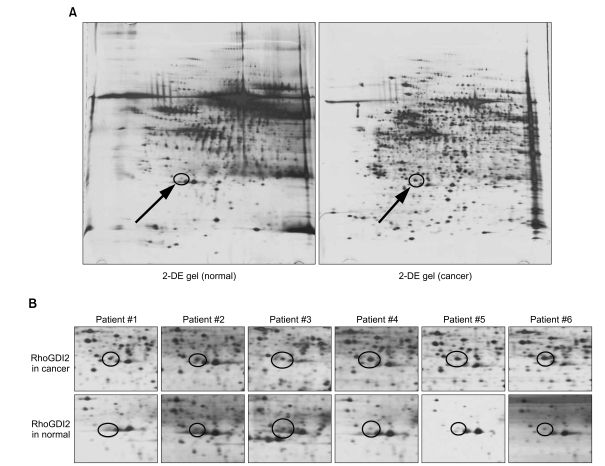
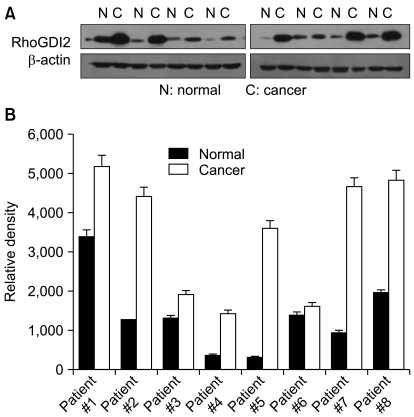
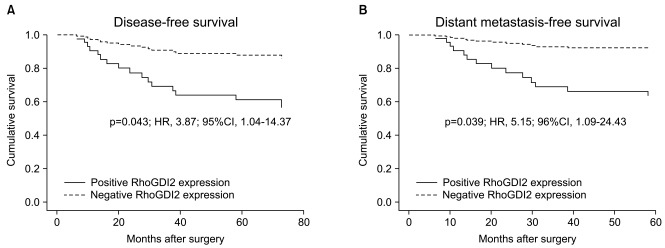
By using a proteomic approach, we observed an increased expression of RhoGDI2 in human breast cancer tissues when compared to that of the normal breast tissues, and we validated its up-regulation in an independent cohort of 8 breast cancer patients. The clinical implication of a RhoGDI2 expression was investigated in 57 breast cancer patients by performing immunohistochemistry. RhoGDI2 did not show a significant association with the tumor size, lymph node metastasis, the histologic grade or the hormone receptor status. However, the patients with RhoGDI2-expressing tumors had significantly shorter disease-free survival (p=0.043; hazard ratio, 3.87) and distant metastasis-free survival (p=0.039; hazard ratio, 5.15).
CONCLUSION
Our results demonstrated a potential role of RhoGDI2 as a poor prognostic marker as well as a potential therapeutic target. The pro-metastatic nature of RhoGDI2 shown in our study may indicate its organ-specific role in cancer metastasis.
MeSH Terms
Figure
Reference
-
1. Weigelt B, Peterse JL, van't Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005; 5:591–602. PMID: 16056258.
Article2. Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat Rev Cancer. 2003; 3:453–458. PMID: 12778135.
Article3. Eccles SA, Welch DR. Metastasis: recent discoveries and novel treatment strategies. Lancet. 2007; 369:1742–1757. PMID: 17512859.
Article4. Steeg PS. Metastasis suppressors alter the signal transduction of cancer cells. Nat Rev Cancer. 2003; 3:55–63. PMID: 12509767.
Article5. Steeg PS, Ouatas T, Halverson D, Palmieri D, Salerno M. Metastasis suppressor genes: basic biology and potential clinical use. Clin Breast Cancer. 2003; 4:51–62. PMID: 12744759.
Article6. DerMardirossian C, Bokoch GM. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 2005; 15:356–363. PMID: 15921909.
Article7. Gildea JJ, Seraj MJ, Oxford G, Harding MA, Hampton GM, Moskaluk CA, et al. RhoGDI2 is an invasion and metastasis suppressor gene in human cancer. Cancer Res. 2002; 62:6418–6423. PMID: 12438227.8. Theodorescu D, Sapinoso LM, Conaway MR, Oxford G, Hampton GM, Frierson HF Jr. Reduced expression of metastasis suppressor RhoGDI2 is associated with decreased survival for patients with bladder cancer. Clin Cancer Res. 2004; 10:3800–3806. PMID: 15173088.
Article9. Moissoglu K, McRoberts KS, Meier JA, Theodorescu D, Schwartz MA. Rho GDP dissociation inhibitor 2 suppresses metastasis via unconventional regulation of RhoGTPases. Cancer Res. 2009; 69:2838–2844. PMID: 19276387.
Article10. Harding MA, Theodorescu D. RhoGDI2: a new metastasis suppressor gene: discovery and clinical translation. Urol Oncol. 2007; 25:401–406. PMID: 17826660.
Article11. Jiang WG, Watkins G, Lane J, Cunnick GH, Douglas-Jones A, Mokbel K, et al. Prognostic value of rho GTPases and rho guanine nucleotide dissociation inhibitors in human breast cancers. Clin Cancer Res. 2003; 9:6432–6440. PMID: 14695145.12. Hu LD, Zou HF, Zhan SX, Cao KM. Biphasic expression of RhoGDI2 in the progression of breast cancer and its negative relation with lymph node metastasis. Oncol Rep. 2007; 17:1383–1389. PMID: 17487395.
Article13. Zhang Y, Zhang B. D4-GDI, a Rho GTPase regulator, promotes breast cancer cell invasiveness. Cancer Res. 2006; 66:5592–5598. PMID: 16740694.
Article14. Jung EJ, Moon HG, Cho BI, Jeong CY, Joo YT, Lee YJ, et al. Galectin-1 expression in cancer-associated stromal cells correlates tumor invasiveness and tumor progression in breast cancer. Int J Cancer. 2007; 120:2331–2338. PMID: 17304502.
Article15. Fritz G, Just I, Kaina B. Rho GTPases are over-expressed in human tumors. Int J Cancer. 1999; 81:682–687. PMID: 10328216.
Article16. Bouzahzah B, Albanese C, Ahmed F, Pixley F, Lisanti MP, Segall JD, et al. Rho family GTPases regulate mammary epithelium cell growth and metastasis through distinguishable pathways. Mol Med. 2001; 7:816–830. PMID: 11844870.
Article17. Jo M, Thomas KS, Somlyo AV, Somlyo AP, Gonias SL. Cooperativity between the Ras-ERK and Rho-Rho kinase pathways in urokinase-type plasminogen activator-stimulated cell migration. J Biol Chem. 2002; 277:12479–12485. PMID: 11805108.
Article18. Miura Y, Kikuchi A, Musha T, Kuroda S, Yaku H, Sasaki T, et al. Regulation of morphology by rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI) in Swiss 3T3 cells. J Biol Chem. 1993; 268:510–515. PMID: 8416955.
Article19. Leffers H, Nielsen MS, Andersen AH, Honoré B, Madsen P, Vandekerckhove J, et al. Identification of two human Rho GDP dissociation inhibitor proteins whose overexpression leads to disruption of the actin cytoskeleton. Exp Cell Res. 1993; 209:165–174. PMID: 8262133.
Article20. Leone A, Seeger RC, Hong CM, Hu YY, Arboleda MJ, Brodeur GM, et al. Evidence for nm23 RNA overexpression, DNA amplification and mutation in aggressive childhood neuroblastomas. Oncogene. 1993; 8:855–865. PMID: 8384356.21. Nakamori S, Ishikawa O, Ohhigashi H, Kameyama M, Furukawa H, Sasaki Y, et al. Expression of nucleoside diphosphate kinase/nm23 gene product in human pancreatic cancer: an association with lymph node metastasis and tumor invasion. Clin Exp Metastasis. 1993; 11:151–158. PMID: 8383029.
Article22. Yokoyama A, Okabe-Kado J, Wakimoto N, Kobayashi H, Sakashita A, Maseki N, et al. Evaluation by multivariate analysis of the differentiation inhibitory factor nm23 as a prognostic factor in acute myelogenous leukemia and application to other hematologic malignancies. Blood. 1998; 91:1845–1851. PMID: 9490665.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Potential of the Microbiome as a Biomarker for Early Diagnosis and Prognosis of Breast Cancer
- Comments on the "Prognostic Impact and Clinicopathological Correlation of CD133 and ALDH1 Expression in Invasive Breast Cancer"
- The Expression of High Mobility Group I (Y) Proteins in Human Breast Cancer
- Prognostic Implications of MicroRNA-21 Overexpression in Invasive Ductal Carcinomas of the Breast
- The Expression of High Mobility Group I (Y) Proteins in Human Breast Cancer