Ann Surg Treat Res.
2015 Mar;88(3):152-159. 10.4174/astr.2015.88.3.152.
Effect of imatinib mesylate and rapamycin on the preformed intimal hyperplasia in rat carotid injury model
- Affiliations
-
- 1Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 2Department of Surgery, Seoul National University College of Medicine, Seoul, Korea. jwhamd@snu.ac.kr
- KMID: 2266873
- DOI: http://doi.org/10.4174/astr.2015.88.3.152
Abstract
- PURPOSE
Intimal hyperplasia (IH) is the main cause of restenosis or occlusion after vascular procedures. Imatinib mesylate and rapamycin are known to prevent IH. The purpose of this study was to evaluate the effect of these drugs on the regression of preformed IH in rat carotid injury model.
METHODS
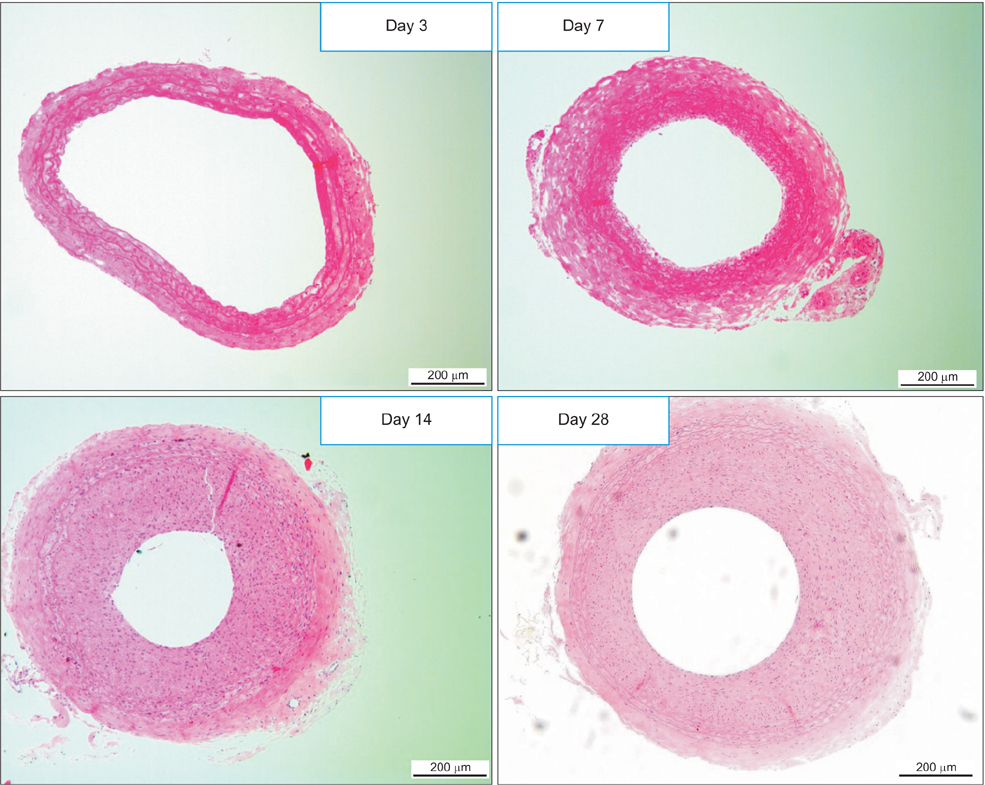
IH was established in rat carotid arteries using a balloon catheter. The drug effects were assessed in vitro on proliferation, migration, and apoptosis of vascular smooth muscle cells (VSMC) in the neointima. And in vivo studies were carried out in 4 groups: imatinib, rapamycin, combined, and no medication. After 2-week oral medication, morphometric analysis evaluated the number and density of neointimal cells, intima-to-media (I/M) ratio and cross-sectional area. Cell proliferation, apoptosis, and collagen changes were also investigated by immunohistochemical staining (IHCS).
RESULTS
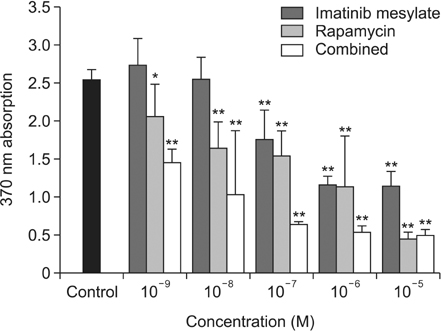
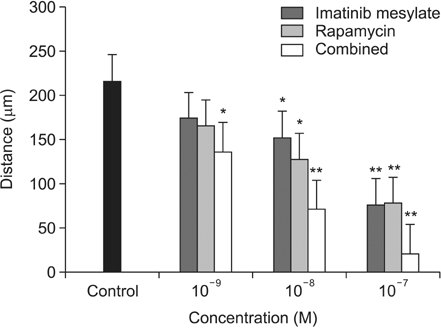
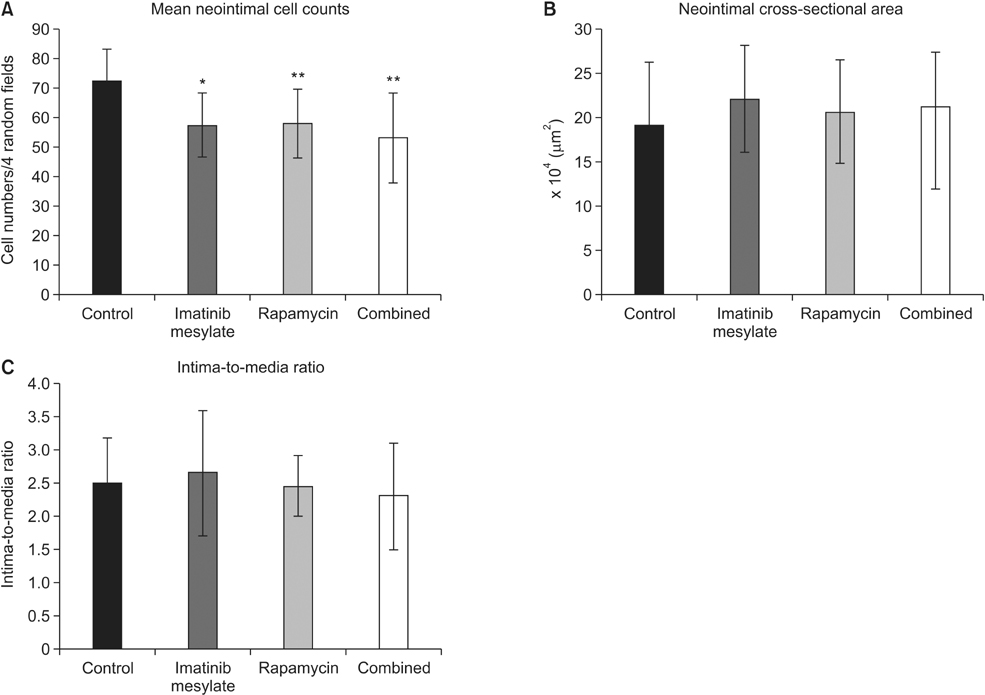
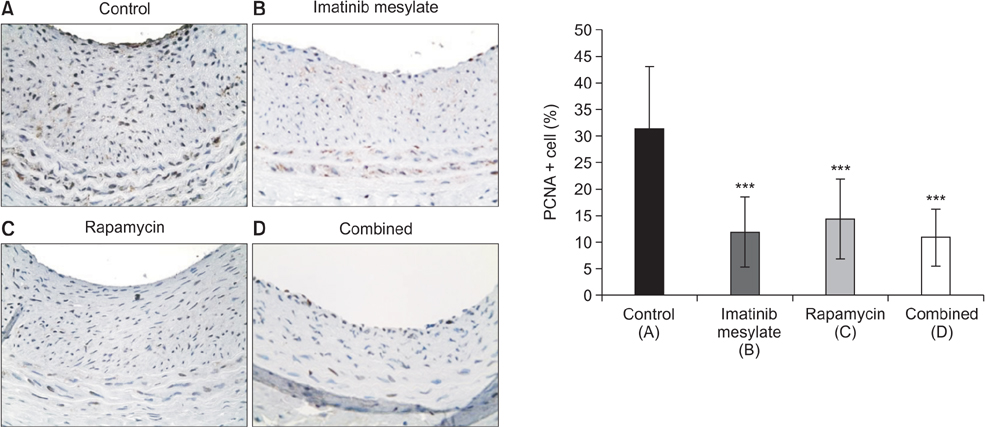
Imatinib and rapamycin significantly inhibited VSMC proliferation and migration, and promoted apoptosis in vitro. In morphometric analysis, the number and density of neointimal cells decreased significantly in all medication groups compared with control group (P < 0.01). However, there was no significant difference in neointimal cross-sectional area and I/M ratio among groups. In IHCS, imatinib and rapamycin inhibited neointimal cell proliferation significantly. However, there was no significant change in cell apoptosis and collagen composition.
CONCLUSION
Combined treatment of with imatinib and rapamycin induced reduction of cell mass in preformed intimal hyperplasia, but failed to induce regression of intimal mass in this short-term medication study. Further studies will be needed with additional strategies of inducing lysis of the extracellular matrix.
Keyword
MeSH Terms
Figure
Reference
-
1. Bauters C, Isner JM. The biology of restenosis. Prog Cardiovasc Dis. 1997; 40:107–116.2. Mitra AK, Gangahar DM, Agrawal DK. Cellular, molecular and immunological mechanisms in the pathophysiology of vein graft intimal hyperplasia. Immunol Cell Biol. 2006; 84:115–124.3. Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003; 349:1315–1323.4. Gregory CR, Huie P, Billingham ME, Morris RE. Rapamycin inhibits arterial intimal thickening caused by both alloimmune and mechanical injury. Its effect on cellular, growth factor, and cytokine response in injured vessels. Transplantation. 1993; 55:1409–1418.5. Buchdunger E, Cioffi CL, Law N, Stover D, Ohno-Jones S, Druker BJ, et al. Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. J Pharmacol Exp Ther. 2000; 295:139–145.6. Wang CH, Anderson N, Li SH, Szmitko PE, Cherng WJ, Fedak PW, et al. Stem cell factor deficiency is vasculoprotective: unraveling a new therapeutic potential of imatinib mesylate. Circ Res. 2006; 99:617–625.7. Sihvola R, Koskinen P, Myllarniemi M, Loubtchenkov M, Hayry P, Buchdunger E, et al. Prevention of cardiac allograft arteriosclerosis by protein tyrosine kinase inhibitor selective for platelet-derived growth factor receptor. Circulation. 1999; 99:2295–2301.8. Lassila M, Allen TJ, Cao Z, Thallas V, Jandeleit-Dahm KA, Candido R, et al. Imainib attenuates diabetes-associated atherosclerosis. Arterioscler Thromb Vasc Biol. 2004; 24:935–942.9. Vamvakopoulos JE, Petrov L, Aavik S, Lehti S, Aavik E, Hayry P. Synergistic suppression of rat neointimal hyperplasia by rapamycin and imatinib mesylate: implications for the prevention of accelerated arteriosclerosis. J Vasc Res. 2006; 43:184–192.10. Park KW, Yang HM, Youn SW, Yang HJ, Chae IH, Oh BH, et al. Constitutively active glycogen synthase kinase-3beta gene transfer sustains apoptosis, inhibits proliferation of vascular smooth muscle cells, and reduces neointima formation after balloon injury in rats. Arterioscler Thromb Vasc Biol. 2003; 23:1364–1369.11. Klouche M, Peri G, Knabbe C, Eckstein HH, Schmid FX, Schmitz G, et al. Modified atherogenic lipoproteins induce expression of pentraxin-3 by human vascular smooth muscle cells. Atherosclerosis. 2004; 175:221–228.12. Johnson C, Galis ZS. Matrix metalloproteinase-2 and -9 differentially regulate smooth muscle cell migration and cell-mediated collagen organization. Arterioscler Thromb Vasc Biol. 2004; 24:54–60.13. Kim HS, Cho HJ, Cho HJ, Park SJ, Park KW, Chae IH, et al. The essential role of p21 in radiation-induced cell cycle arrest of vascular smooth muscle cell. J Mol Cell Cardiol. 2004; 37:871–880.14. Gown AM, Willingham MC. Improved detection of apoptotic cells in archival paraffin sections: immunohistochemistry using antibodies to cleaved caspase 3. J Histochem Cytochem. 2002; 50:449–454.15. Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979; 11:447–455.16. Puchtler H, Waldrop FS, Valentine LS. Polarization microscopic studies of connective tissue stained with picro-sirius red FBA. Beitr Pathol. 1973; 150:174–187.17. Suh JW, Park JS, Cho HJ, Kim MS, Kang HJ, Cho YS, et al. Sirolimus-eluting stent showed better one-year outcomes than paclitaxel-eluting stent in a real life setting of coronary intervention in Koreans. Int J Cardiol. 2007; 117:31–36.18. Stone GW, Ellis SG, Cannon L, Mann JT, Greenberg JD, Spriggs D, et al. Comparison of a polymer-based paclitaxel-eluting stent with a bare metal stent in patients with complex coronary artery disease: a randomized controlled trial. JAMA. 2005; 294:1215–1223.19. Dawkins KD, Grube E, Guagliumi G, Banning AP, Zmudka K, Colombo A, et al. Clinical efficacy of polymer-based paclitaxel-eluting stents in the treatment of complex, long coronary artery lesions from a multicenter, randomized trial: support for the use of drug-eluting stents in contemporary clinical practice. Circulation. 2005; 112:3306–3313.20. Min SK, Kenagy RD, Clowes AW. Induction of vascular atrophy as a novel approach to treating restenosis. A review. J Vasc Surg. 2008; 47:662–670.21. Vallieres K, Petitclerc E, Laroche G. On the ability of imatinib mesylate to inhibit smooth muscle cell proliferation without delaying endothelialization: an in vitro study. Vascul Pharmacol. 2009; 51:50–56.22. Matter CM, Rozenberg I, Jaschko A, Greutert H, Kurz DJ, Wnendt S, et al. Effects of tacrolimus or sirolimus on proliferation of vascular smooth muscle and endothelial cells. J Cardiovasc Pharmacol. 2006; 48:286–292.23. Fandrich F, Ruhnke M, Dresske B, Kremer B. Tolerance-inducing strategies in transplantation surgery-current status and perspectives. Langenbecks Arch Surg. 2004; 389:60–66.24. Nasuno A, Toba K, Ozawa T, Hanawa H, Osman Y, Hotta Y, et al. Expression of coxsackievirus and adenovirus receptor in neointima of the rat carotid artery. Cardiovasc Pathol. 2004; 13:79–84.25. Ruygrok PN, Webber B, Faddy S, Muller DW, Keogh A. Angiographic regression of cardiac allograft vasculopathy after introducing sirolimus immunosuppression. J Heart Lung Transplant. 2003; 22:1276–1279.26. Uitto J, Kouba D. Cytokine modulation of extracellular matrix gene expression: relevance to fibrotic skin diseases. J Dermatol Sci. 2000; 24:Suppl 1. S60–S69.27. Distler JH, Jungel A, Huber LC, Schulze-Horsel U, Zwerina J, Gay RE, et al. Imatinib mesylate reduces production of extracellular matrix and prevents development of experimental dermal fibrosis. Arthritis Rheum. 2007; 56:311–322.28. Zhu J, Wu J, Frizell E, Liu SL, Bashey R, Rubin R, et al. Rapamycin inhibits hepatic stellate cell proliferation in vitro and limits fibrogenesis in an in vivo model of liver fibrosis. Gastroenterology. 1999; 117:1198–1204.29. Poulalhon N, Farge D, Roos N, Tacheau C, Neuzillet C, Michel L, et al. Modulation of collagen and MMP-1 gene expression in fibroblasts by the immunosuppressive drug rapamycin. A direct role as an antifibrotic agent? J Biol Chem. 2006; 281:33045–33052.30. Abdollahi A, Li M, Ping G, Plathow C, Domhan S, Kiessling F, et al. Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. J Exp Med. 2005; 201:925–935.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibition of intimal hyperplasia by local perivascular application of rapamycin and imatinib mesilate after carotid balloon injury
- Different Responses of Neointimal Cells to Imatinib Mesylate and Rapamycin Compared with Normal Vascular Smooth Muscle Cells
- A Case of Lichenoid Drug Eruption Associated with Imatinib Mesylate
- Perivascular Delivery of Rapamycin in Pluronic Gel Inhibits Neointimal Hyperplasia in a Rat Carotid Artery Injury Model, and the Complementary Role of Carotid Arteriography
- Intimal Hyperplasia in Loop-Injured Carotid Arteries Is Attenuated in Transglutaminase 2-Null Mice