Korean Circ J.
2008 Feb;38(2):80-86. 10.4070/kcj.2008.38.2.80.
Perivascular Delivery of Rapamycin in Pluronic Gel Inhibits Neointimal Hyperplasia in a Rat Carotid Artery Injury Model, and the Complementary Role of Carotid Arteriography
- Affiliations
-
- 1Department of Pediatrics, Gil Heart Center, Incheon, Korea.
- 2Department of Internal Medicine, College of Medicine, Chonnam National University, Gwangju, Korea.
- 3Division of Cardiology, Department of Internal Medicine, College of Medicine, Chungbuk National University, Cheongju, Korea. kdwoon@chungbuk.ac.kr
- KMID: 2225840
- DOI: http://doi.org/10.4070/kcj.2008.38.2.80
Abstract
- BACKGROUND AND OBJECTIVES
Rapamycin has been shown to inhibit the vascular smooth muscle cell migration and proliferation that contributes to neointimal formation. We investigated whether the perivascular delivery of rapamycin in Pluronic gel could inhibit neointimal hyperplasia in a rat carotid artery model, and we tested the usefulness of carotid arteriography.
MATERIALS AND METHODS
To assess the kinetics of rapamycin's release from Pluronic gel, a [3H] thymidine incorporation assay was performed with using the media exposed to rapamycin in Pluronic gel for 10, 20, 60 and 120 min. We applied 100 microgram of rapamycin in Pluronic gel to the perivascular space of the carotid artery after the balloon injury (n=9), whereas only gel was applied in a control group (n=9). We performed the carotid arteriography and the morphometric analysis 14 days after injury.
RESULTS
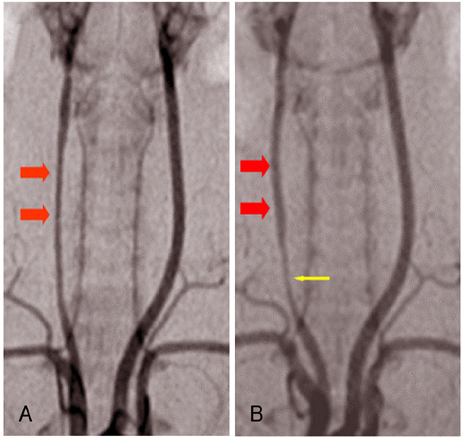
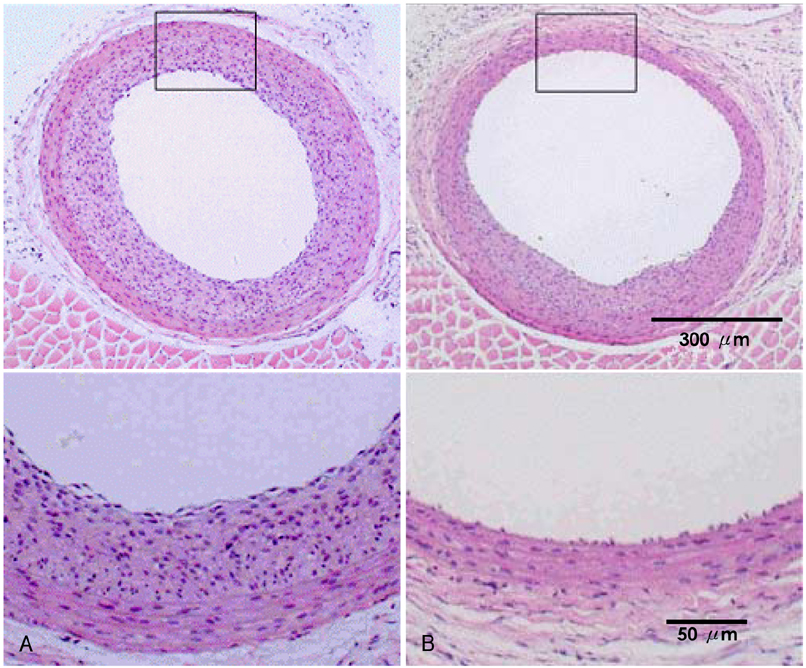
The [3H] thymidine incorporation assay showed a reduction of uptake in a time-dependent manner (86%, 48%, 45% and 40% of the control, respectively, at 10, 20, 60 and 120 minutes). The inhibiting effect of rapamycin on neointimal hyperplasia was identified on the carotid arteriography (mean luminal diameter; 0.75+/-0.11 vs. 0.60+/-0.12 arbitrary units, respectively, p< 0.05) and on the morphometric analysis (neointima area: 0.09+/-0.03 vs. 0.17+/-0.06 mm(2), respectively, p< 0.05).
CONCLUSION
This study demonstrated that perivascular delivery of rapamycin in Pluronic gel inhibits neointimal hyperplasia in a rat carotid injury model. This animal model combined with arteriography can be used for developing new drugs to treat restenosis. In addition, this technique might be useful for vascular surgery such as coronary artery bypass grafting, arteriovenous fistula formation and peripheral vascular bypass graft insertion.
Keyword
MeSH Terms
Figure
Reference
-
1. Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993. 362:801–809.2. Chen D, Krasinski K, Sylvester A, Chen J, Nisen PD, Andrés V. Down regulation of cyclin-dependent kinase 2 activity and cyclin A promoter activity in vascular smooth muscle cells by p27(KIP1), an inhibitor of neointima formation in the rat carotid artery. J Clin Invest. 1997. 99:2334–2341.3. Waltenberger J. Modulation of growth factor action: implications for the treatment of cardiovascular diseases. Circulation. 1997. 96:4083–4094.4. Gordon D, Reidy MA, Benditt EP, Schwartz SM. Cell proliferation on human coronary arteries. Proc Natl Acad Sci U S A. 1990. 87:4600–4604.5. Mintz GS, Popma JJ, Pichard AD, et al. Arterial remodeling after coronary angioplasty: a serial intravascular ultrasound study. Circulation. 1996. 94:35–43.6. Sriram V, Patterson C. Cell cycle in vasculoproliferative diseases: potential interventions and routes of delivery. Circulation. 2001. 103:2414–2419.7. Fattori R, Piva T. Drug-eluting stents in vascular intervention. Lancet. 2003. 361:247–249.8. Pearce BJ, McKinsey JF. Current status of intravascular stents as delivery devices to prevent restenosis. Vasc Endovascular Surg. 2003. 37:231–237.9. Kataoka T, Honda Y, Bonneau HN, Yock PG, Fitzerald PJ. New catheter-based technology for the treatment of restenosis. J Interv Cardiol. 2002. 15:371–379.10. Kim DW, Kwon JS, Kim YG, et al. Novel oral formulation of paclitaxel inhibits neointimal hyperplasia in a rat carotid artery injury model. Circulation. 2004. 109:1558–1563.11. Cho MC, Kwak NJ, Piao H, et al. Effect of paclitaxel local delivery on neointimal formation after endothelial denudation of the rat carotid artery. Korean Cire J. 2000. 30:198–207.12. Kwon JS, Park SS, Kim YG, et al. Perivascular delivery of paclitaxel with F-127 Pluronic gel inhibits neointimal hyperplasia in a rat carotid artery injury model. Korean Circ J. 2005. 35:221–227.13. Kim DW, Kim YG, Oh TG, Cho MC, Kim ST. Retrovirus-mediated herpes simplex virus kinase gene therapy for the prevention of stenosis in rat carotid artery injury model. Korean Circ J. 1998. 28:977–989.14. Cagiannos C, Abul-Khoudoud OR, DeRijk W, et al. Rapamycin-coated expanded polytetrafluoroethylene bypass grafts exhibit decreased anastomotic neointimal hyperplasia in a porcine model. J Vasc Surg. 2005. 42:980–988.15. Gallo R, Padurean A, Jayaraman T, et al. Inhibition of intimal thickening after balloon angioplasty in porcine coronary arteries by targeting regulators of the cell cycle. Circulation. 1999. 99:2164–2170.16. Suzuki T, Kopia G, Hayashi S, et al. Stent-based delivery of rapamycin reduces neointimal formation in a porcine coronary model. Circulation. 2001. 104:1188–1193.17. Pires NMM, van der Hoeven BL, de Vries MR, et al. Local perivascular delivery of anti-restenotic agents from a drug-eluting poly (ε-caprolactone) stent cuff. Biomaterials. 2005. 26:5386–5394.18. Fukuda D, Sata M, Tanaka K, Nagai R. Potent inhibitory effect of sirolimus on circulating vascular progenitor cells. Circulation. 2005. 111:926–931.19. Sousa JE, Costa MA, Abizaid A, et al. Sirolimus-eluting stent for the treatment of in-stent restenosis: a quantitative coronary angiography and three-dimensional intravascular ultrasound study. Circulation. 2003. 107:24–27.20. Shafiq N, Malhotra S, Pandhi P, Grover A, Uboweja A. A meta-analysis of clinical trials of paclitaxel- and sirolimus-eluting stents in patients with obstructive coronary artery disease. Br J Clin Pharmacol. 2005. 59:94–101.21. Signore PE, Machan LS, Jackson JK, et al. Complete inhibition of intimal hyperplasia by perivascular delivery of paclitaxel in balloon-injured rat carotid arteries. J Vasc Interv Radiol. 2001. 12:79–88.22. Schachner T, Zou Y, Oberhuber A, et al. Local application of rapamycin inhibits neointimal hyperplasia in experimental vein grafts. Ann Thorac Surg. 2004. 77:1580–1585.23. Hafizi S, Mordi VN, Andersson KM, Chester AH, Yacoub MH. Differential effects of rapamycin, cyclosporine A, and FK506 on human coronary artery smooth muscle cell proliferation and signalling. Vascul Pharmacol. 2004. 41:167–176.24. Rotmans JI, Pattynama PM, Verhagen HJ, et al. Sirolimus-eluting stents to abolish intimal hyperplasia and improve flow in porcine arteriovenous grafts: a 4-week follow-up study. Circulation. 2005. 111:1537–1542.25. Hu Y, Zou Y, Dietrich H, Wick G, Xu Q. Inhibition of neointimal hyperplasia of mouse vein grafts by locally applied suramin. Circulation. 1999. 100:861–868.26. Omura T, Yoshiyama M, Izumi Y, et al. Involvement of c-Jun NH2 terminal kinase and p38MAPK in rapamycin-mediated inhibition of neointimal formation in rat carotid arteries. J Cardiovasc Pharmacol. 2005. 46:519–525.27. Scott NA, Cipolla GD, Ross CE, et al. Identification of a potential role for the adventitia in vascular lesion formation after balloon overstretch injury of porcine coronary arteries. Circulation. 1996. 93:2178–2187.28. Currier JW, Faxon DP. Restenosis after percutaneous transluminal coronary angioplasty: have we been aiming at the wrong target? J Am Coll Cardiol. 1995. 25:516–520.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Perivascular Delivery of Paclitaxel with F-127 Pluronic Gel Inhibits Neointimal Hyperplasia in a Rat Carotid Artery Injury Model
- Inhibition of intimal hyperplasia by local perivascular application of rapamycin and imatinib mesilate after carotid balloon injury
- Retrovirus-Mediated Herpes Simplex Virus Thymidine Kinase Gene Therapy for the Prevention of Stenosis in Rat Carotid Artery Injury Model
- Effect of Local Administration of Lovastatin on Preventing Neointimal Hyperplasia in the Rat Carotid Artery Injury Model
- Effect of Local Administration of Lovastatin on Preventing Neointimal Hyperplasia in the Rat Carotid Artery Injury Model