Ann Rehabil Med.
2013 Dec;37(6):766-776. 10.5535/arm.2013.37.6.766.
Randomized, Sham Controlled Trial of Transcranial Direct Current Stimulation for Painful Diabetic Polyneuropathy
- Affiliations
-
- 1Department of Rehabilitation Medicine, Eulji Hospital, Eulji University School of Medicine, Seoul, Korea. md52516@hanmail.net
- 2Department of Biomedical Engineering, Keimyung University, Daegu, Korea.
- 3Department of Biostatistics, Yonsei University College of Medicine, Seoul, Korea.
- 4Department of Medicine, Eulji Hospital, Eulji University School of Medicine, Seoul, Korea.
- KMID: 2266555
- DOI: http://doi.org/10.5535/arm.2013.37.6.766
Abstract
OBJECTIVE
To investigate the analgesic effect of transcranial direct current stimulation (tDCS) over the primary motor (M1), dorsolateral prefrontal cortex (DLPFC), and sham tDCS in patients with painful diabetic polyneuropathy (PDPN).
METHODS
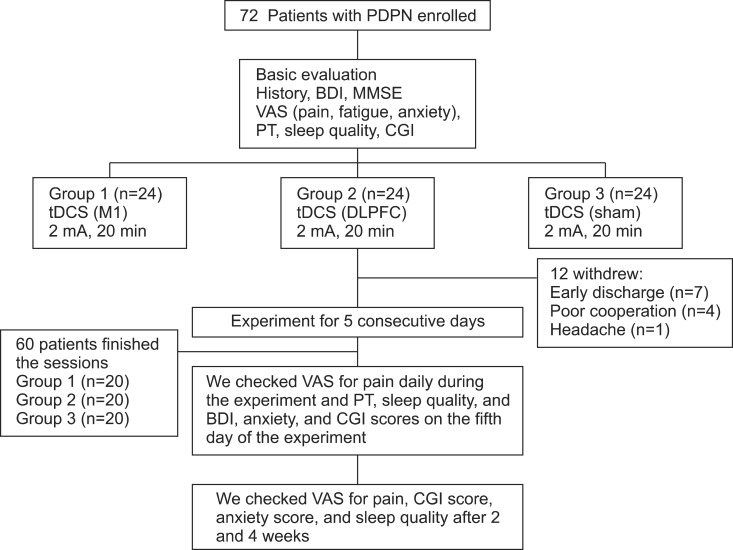
Patients with PDPN (n=60) were divided randomly into the three groups (n=20 per group). Each group received anodal tDCS with the anode centered over the left M1, DLPFC, or sham stimulation for 20 minutes at intensity of 2 mA for 5 consecutive days. A blinded physician rated the patients' pain using a visual analog scale (VAS), Clinical Global Impression (CGI) score, anxiety score, sleep quality, Beck Depression Inventory (BDI), and the pain threshold (PT) to pressure.
RESULTS
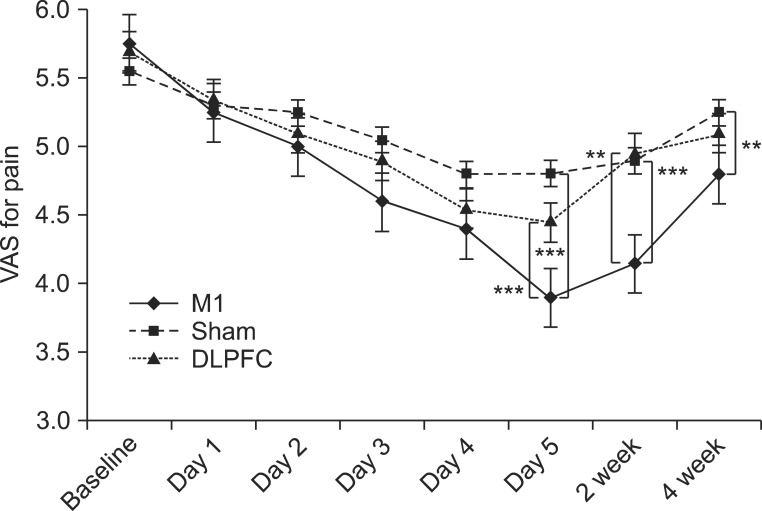
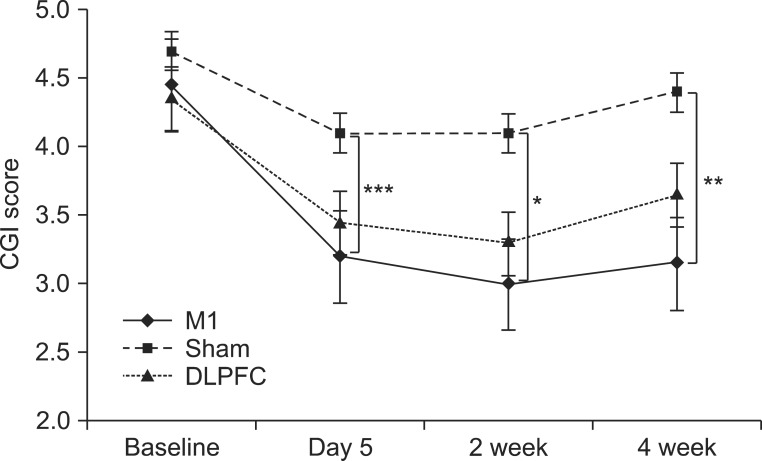
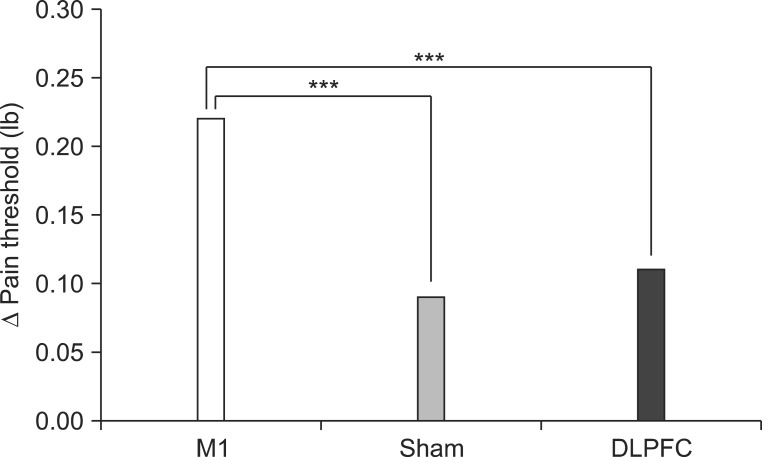
After the tDCS sessions, the M1 group showed a significantly greater reduction in VAS for pain and PT versus the sham and DLPFC groups (p<0.001). The reduction in VAS for pain was sustained after 2 and 4 weeks of follow-up in the M1 group compared with the sham group (p<0.001, p=0.007). Significant differences were observed among the three groups over time in VAS for pain (p<0.001), CGI score (p=0.01), and PT (p<0.001). No significant difference was observed among the groups in sleep quality, anxiety score, or BDI score immediately after tDCS.
CONCLUSION
Five daily sessions of tDCS over the M1 can produce immediate pain relief, and relief 2- and 4-week in duration in patients with PDPN. Our findings provide the first evidence of a beneficial effect of tDCS on PDPN.
MeSH Terms
Figure
Reference
-
1. Boulton AJ, Malik RA, Arezzo JC, Sosenko JM. Diabetic somatic neuropathies. Diabetes Care. 2004; 27:1458–1486. PMID: 15161806.
Article2. Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care. 2006; 29:1518–1522. PMID: 16801572.
Article3. Jensen MP, Chodroff MJ, Dworkin RH. The impact of neuropathic pain on health-related quality of life: review and implications. Neurology. 2007; 68:1178–1182. PMID: 17420400.
Article4. Tesfaye S, Chaturvedi N, Eaton SE, Ward JD, Manes C, Ionescu-Tirgoviste C, et al. Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005; 352:341–350. PMID: 15673800.
Article5. Baron R. Mechanisms of disease: neuropathic pain: a clinical perspective. Nat Clin Pract Neurol. 2006; 2:95–106. PMID: 16932531.6. Fischer TZ, Waxman SG. Neuropathic pain in diabetes: evidence for a central mechanism. Nat Rev Neurol. 2010; 6:462–466. PMID: 20625378.7. Selvarajah D, Wilkinson ID, Emery CJ, Harris ND, Shaw PJ, Witte DR, et al. Early involvement of the spinal cord in diabetic peripheral neuropathy. Diabetes Care. 2006; 29:2664–2669. PMID: 17130202.
Article8. Eaton SE, Harris ND, Rajbhandari SM, Greenwood P, Wilkinson ID, Ward JD, et al. Spinal-cord involvement in diabetic peripheral neuropathy. Lancet. 2001; 358:35–36. PMID: 11454377.
Article9. Selvarajah D, Wilkinson ID, Emery CJ, Shaw PJ, Griffiths PD, Gandhi R, et al. Thalamic neuronal dysfunction and chronic sensorimotor distal symmetrical polyneuropathy in patients with type 1 diabetes mellitus. Diabetologia. 2008; 51:2088–2092. PMID: 18773192.
Article10. Fischer TZ, Tan AM, Waxman SG. Thalamic neuron hyperexcitability and enlarged receptive fields in the STZ model of diabetic pain. Brain Res. 2009; 1268:154–161. PMID: 19285053.
Article11. Fregni F, Freedman S, Pascual-Leone A. Recent advances in the treatment of chronic pain with non-invasive brain stimulation techniques. Lancet Neurol. 2007; 6:188–191. PMID: 17239806.
Article12. Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006; 117:845–850. PMID: 16427357.
Article13. Fregni F, Boggio PS, Lima MC, Ferreira MJ, Wagner T, Rigonatti SP, et al. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain. 2006; 122:197–209. PMID: 16564618.
Article14. Mori F, Codeca C, Kusayanagi H, Monteleone F, Buttari F, Fiore S, et al. Effects of anodal transcranial direct current stimulation on chronic neuropathic pain in patients with multiple sclerosis. J Pain. 2010; 11:436–442. PMID: 20018567.
Article15. Khedr EM, Kotb H, Kamel NF, Ahmed MA, Sadek R, Rothwell JC. Longlasting antalgic effects of daily sessions of repetitive transcranial magnetic stimulation in central and peripheral neuropathic pain. J Neurol Neurosurg Psychiatry. 2005; 76:833–838. PMID: 15897507.16. Fregni F, Boggio PS, Nitsche MA, Marcolin MA, Rigonatti SP, Pascual-Leone A. Treatment of major depression with transcranial direct current stimulation. Bipolar Disord. 2006; 8:203–204. PMID: 16542193.
Article17. Boggio PS, Zaghi S, Lopes M, Fregni F. Modulatory effects of anodal transcranial direct current stimulation on perception and pain thresholds in healthy volunteers. Eur J Neurol. 2008; 15:1124–1130. PMID: 18717717.
Article18. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010; 33:S62–S69. PMID: 20042775.19. Bastyr EJ 3rd, Price KL, Bril V. MBBQ Study Group. Development and validity testing of the neuropathy total symptom score-6: questionnaire for the study of sensory symptoms of diabetic peripheral neuropathy. Clin Ther. 2005; 27:1278–1294. PMID: 16199253.
Article20. Fregni F, Gimenes R, Valle AC, Ferreira MJ, Rocha RR, Natalle L, et al. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum. 2006; 54:3988–3998. PMID: 17133529.
Article21. Roizenblatt S, Fregni F, Gimenez R, Wetzel T, Rigonatti SP, Tufik S, et al. Site-specific effects of transcranial direct current stimulation on sleep and pain in fibromyalgia: a randomized, sham-controlled study. Pain Pract. 2007; 7:297–306. PMID: 17986164.
Article22. Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont). 2007; 4:28–37. PMID: 20526405.23. Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996; 67:588–597. PMID: 8991972.24. Rossi S, Cappa SF, Babiloni C, Pasqualetti P, Miniussi C, Carducci F, et al. Prefrontal [correction of Prefontal] cortex in long-term memory: an "interference" approach using magnetic stimulation. Nat Neurosci. 2001; 4:948–952. PMID: 11528428.25. Herwig U, Satrapi P, Schonfeldt-Lecuona C. Using the international 10-20 EEG system for positioning of transcranial magnetic stimulation. Brain Topogr. 2003; 16:95–99. PMID: 14977202.
Article26. Reidler JS, Mendonca ME, Santana MB, Wang X, Lenkinski R, Motta AF, et al. Effects of motor cortex modulation and descending inhibitory systems on pain thresholds in healthy subjects. J Pain. 2012; 13:450–458. PMID: 22515945.
Article27. Poreisz C, Boros K, Antal A, Paulus W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res Bull. 2007; 72:208–214. PMID: 17452283.
Article28. Rasche D, Ruppolt M, Stippich C, Unterberg A, Tronnier VM. Motor cortex stimulation for long-term relief of chronic neuropathic pain: a 10 year experience. Pain. 2006; 121:43–52. PMID: 16480828.
Article29. Shin HC, Chapin JK. Mapping the effects of motor cortex stimulation on somatosensory relay neurons in the rat thalamus: direct responses and afferent modulation. Brain Res Bull. 1990; 24:257–265. PMID: 2322860.30. Yoo WK, You SH, Ko MH, Kim ST, Park CH, Park JW, et al. High frequency rTMS modulation of the sensorimotor networks: behavioral changes and fMRI correlates. Neuroimage. 2008; 39:1886–1895. PMID: 18086536.
Article31. Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001; 57:1899–1901. PMID: 11723286.
Article32. Lang N, Siebner HR, Ward NS, Lee L, Nitsche MA, Paulus W, et al. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? Eur J Neurosci. 2005; 22:495–504. PMID: 16045502.
Article33. Lefaucheur JP, Drouot X, Keravel Y, Nguyen JP. Pain relief induced by repetitive transcranial magnetic stimulation of precentral cortex. Neuroreport. 2001; 12:2963–2965. PMID: 11588611.
Article34. Antal A, Kriener N, Lang N, Boros K, Paulus W. Cathodal transcranial direct current stimulation of the visual cortex in the prophylactic treatment of migraine. Cephalalgia. 2011; 31:820–828. PMID: 21398419.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Self-administered Transcranial Direct Stimulation in Patients with Major Depressive Disorder: A Randomized, Single-blinded Clinical Trial
- Effects of Transcranial Direct-Current Stimulation Therapy on Primary Chronic Insomnia: A Proof-of Concept Clinical Trial
- Efficacy of Unilateral and Bilateral Parietal Transcranial Direct Current Stimulation on Right Hemispheric Stroke Patients With Neglect Symptoms: A Proof-of-Principle Study
- Application of Transcranial Direct Current Stimulation in Psychiatry
- Non-Invasive Brain Stimulation for Treatment of Focal Hand Dystonia: Update and Future Direction