Allergy Asthma Respir Dis.
2014 Jul;2(3):200-207. 10.4168/aard.2014.2.3.200.
The safety and efficacy of recombinant fibroblast growth factor 2 in human asthmatics: A pilot study
- Affiliations
-
- 1Department of Internal Medicine, Dankook University College of Medicine, Cheonan, Korea. ykjee@dankook.ac.kr
- 2Dong-A Pharm. Co. Ltd., Seoul, Korea.
- KMID: 2262520
- DOI: http://doi.org/10.4168/aard.2014.2.3.200
Abstract
- PURPOSE
Fibroblast growth factor 2 (FGF2) has been shown to inhibit airway inflammation, mucus production, and airway hyperresponsiveness in mouse model of asthma. The aim of this study was to evaluate the safety and efficacy of inhaled recombinant FGF2 in asthmatic patients.
METHODS
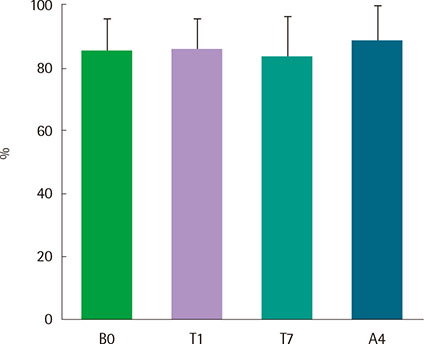
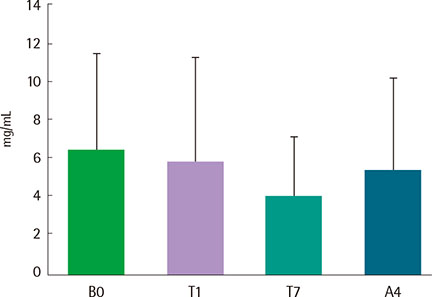
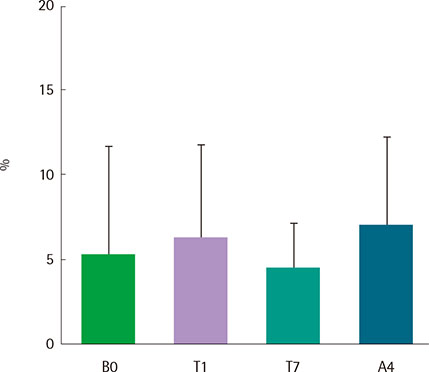
Eight asthmatics were eligible for the study. All patients were admitted to a hospital, and recombinant FGF2 was administered using a nebulizer at a concentration of 4.5 ng/mL three times a day for one week. Pulmonary function test, methacholine bronchial provocation test, induced sputum analysis, asthma control test (ACT), and asthma quality of life questionnaire (AQLQ) were performed at the beginning of wash-out period, before and after the treatment, and at the end of study. And all these parameters were compared before and after FGF2 treatment.
RESULTS
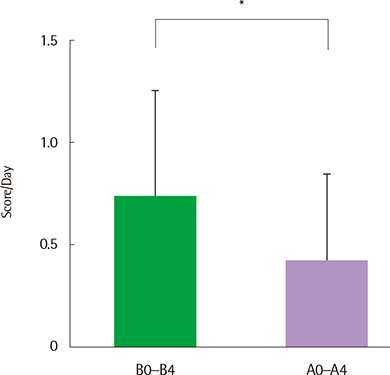
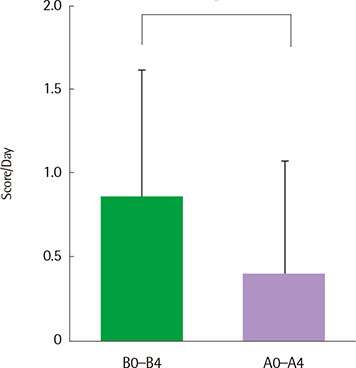
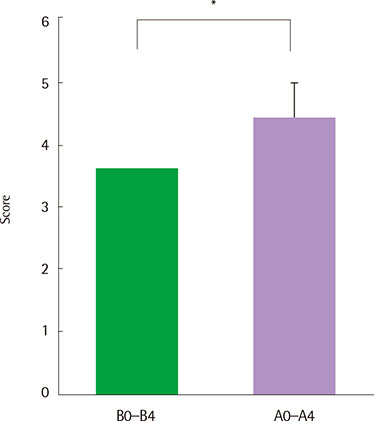
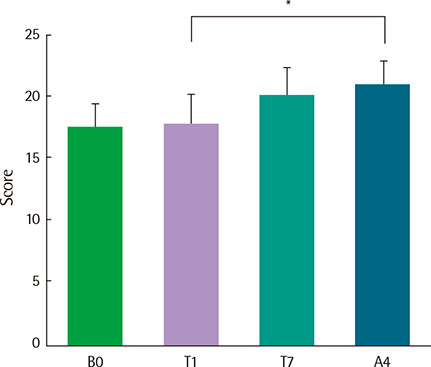
There were no serious adverse events associated with recombinant FGF2 during five-week study period. Daytime and nocturnal symptoms improved after the treatment (P=0.028 and P=0.012, respectively). AQLQ and ACT also improved after the treatment (P=0.017 and P=0.011, respectively). However, lung function, airway hyperresponsiveness, and airway inflammation showed no significant difference before and after the treatment.
CONCLUSION
Inhaled recombinant FGF2 was safely used to eight asthmatics without any serious adverse events, and improved daytime and nocturnal symptoms, and quality of life in adult asthmatics. FGF2 may be a potential drug in the treatment of asthma.
MeSH Terms
-
Adult
Airway Remodeling
Animals
Asthma
Bronchial Provocation Tests
Fibroblast Growth Factor 2*
Humans
Inflammation
Lung
Methacholine Chloride
Mice
Mucus
Nebulizers and Vaporizers
Pilot Projects*
Quality of Life
Respiratory Function Tests
Sputum
Surveys and Questionnaires
Fibroblast Growth Factor 2
Methacholine Chloride
Figure
Reference
-
1. Yamauchi K, Inoue H. Airway remodeling in asthma and irreversible airflow limitation-ECM deposition in airway and possible therapy for remodeling-. Allergol Int. 2007; 56:321–329.
Article2. Sumi Y, Hamid Q. Airway remodeling in asthma. Allergol Int. 2007; 56:341–348.
Article3. Yamauchi K. Airway remodeling in asthma and its influence on clinical pathophysiology. Tohoku J Exp Med. 2006; 209:75–87.
Article4. Mason IJ. The ins and outs of fibroblast growth factors. Cell. 1994; 78:547–552.
Article5. Burgess JK. The role of the extracellular matrix and specific growth factors in the regulation of inflammation and remodelling in asthma. Pharmacol Ther. 2009; 122:19–29.
Article6. Hoshino M, Takahashi M, Aoike N. Expression of vascular endothelial growth factor, basic fibroblast growth factor, and angiogenin immunoreactivity in asthmatic airways and its relationship to angiogenesis. J Allergy Clin Immunol. 2001; 107:295–301.
Article7. Redington AE, Roche WR, Madden J, Frew AJ, Djukanovic R, Holgate ST, et al. Basic fibroblast growth factor in asthma: measurement in bronchoalveolar lavage fluid basally and following allergen challenge. J Allergy Clin Immunol. 2001; 107:384–387.
Article8. Shute JK, Solic N, Shimizu J, McConnell W, Redington AE, Howarth PH. Epithelial expression and release of FGF-2 from heparan sulphate binding sites in bronchial tissue in asthma. Thorax. 2004; 59:557–562.
Article9. Jeon SG, Lee CG, Oh MH, Chun EY, Gho YS, Cho SH, et al. Recombinant basic fibroblast growth factor inhibits the airway hyperresponsiveness, mucus production, and lung inflammation induced by an allergen challenge. J Allergy Clin Immunol. 2007; 119:831–837.
Article10. Kwon HS, Lee SH, Yang MS, Lee SM, Kim SH, Kim DI, et al. Correlation between the Korean version of asthma control test and health-related quality of life in adult asthmatics. J Korean Med Sci. 2008; 23:621–627.
Article11. Chai H, Farr RS, Froehlich LA, Mathison DA, McLean JA, Rosenthal RR, et al. Standardization of bronchial inhalation challenge procedures. J Allergy Clin Immunol. 1975; 56:323–327.
Article12. Pizzichini E, Pizzichini MM, Efthimiadis A, Evans S, Morris MM, Squillace D, et al. Indices of airway inflammation in induced sputum: reproducibility and validity of cell and fluid-phase measurements. Am J Respir Crit Care Med. 1996; 154(2 Pt 1):308–317.
Article13. Jeon SG, Lee SY, Park HY, Kim TB, Kim YK, Cho SH, et al. FGF2 inhibits the sensitization to aeroallergen enhanced by LPS and dsRNA [abstract]. Korean J Med. 2004; 67:Suppl 1. S251. Abstract no. Sat-174.14. Park HK, Kim TB, Jeon SG, Kim YK, Cho SH, Min KU, et al. Allergic reaction enhanced by LPS induces severe lung parenchymal inflammation and alveolar destruction which are inhibited by FGF2 [abstract]. Korean J Med. 2004; 67:Suppl 1. S156. Abstract no. 179.15. Spyrou GE, Naylor IL. The effect of basic fibroblast growth factor on scarring. Br J Plast Surg. 2002; 55:275–282.
Article16. Ito T, Sawada R, Fujiwara Y, Seyama Y, Tsuchiya T. FGF-2 suppresses cellular senescence of human mesenchymal stem cells by down-regulation of TGF-beta2. Biochem Biophys Res Commun. 2007; 359:108–114.
Article17. Cushing MC, Mariner PD, Liao JT, Sims EA, Anseth KS. Fibroblast growth factor represses Smad-mediated myofibroblast activation in aortic valvular interstitial cells. FASEB J. 2008; 22:1769–1777.
Article18. Silverio-Ruiz KG, Martinez AE, Garlet GP, Barbosa CF, Silva JS, Cicarelli RM, et al. Opposite effects of bFGF and TGF-beta on collagen metabolism by human periodontal ligament fibroblasts. Cytokine. 2007; 39:130–137.
Article19. Li CM, Khosla J, Pagan I, Hoyle P, Sannes PL. TGF-beta1 and fibroblast growth factor-1 modify fibroblast growth factor-2 production in type II cells. Am J Physiol Lung Cell Mol Physiol. 2000; 279:L1038–L1046.20. Giannouli CC, Kletsas D. TGF-beta regulates differentially the proliferation of fetal and adult human skin fibroblasts via the activation of PKA and the autocrine action of FGF-2. Cell Signal. 2006; 18:1417–1429.
Article21. Kim TB, Kim YK, Lee CG, Zhu Z, Cho SH, Min KU, et al. FGF2 plays a critical role in airway hyporesponsiveness in TGF-b1 transgenic(+) mice [abstract]. Korean J Med. 2004; 67:Suppl 1. S155. Abstract no. 178.22. Zuany-Amorim C, Haile S, Leduc D, Dumarey C, Huerre M, Vargaftig BB, et al. Interleukin-10 inhibits antigen-induced cellular recruitment into the airways of sensitized mice. J Clin Invest. 1995; 95:2644–2651.
Article23. Tournoy KG, Kips JC, Pauwels RA. Endogenous interleukin-10 suppresses allergen-induced airway inflammation and nonspecific airway responsiveness. Clin Exp Allergy. 2000; 30:775–783.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- ERRATUM: Author's Name Correction. The safety and efficacy of recombinant fibroblast growth factor 2 in human asthmatics: A pilot study
- Effects of Recombinant Human Epidermal Growth Factor on the Proliferation and Radiation Survival of Human Fibroblast Cell Lines in Vitro
- A Study of Pilot-Efficacy
- An Experimental Study of the Effect of Acidic Fibroblast Growth Factor on Nerve Regeneration
- Pfeiffer Syndrome Type 2 with Sporadic Fibroblast Growth Factor Receptor 2 Mutation and Coccygeal Anomaly