Allergy Asthma Respir Dis.
2014 Sep;2(4):259-265. 10.4168/aard.2014.2.4.259.
The effect of heat treatment or hydrolysis on cow's milk protein distributions and antigenicities
- Affiliations
-
- 1Department of Pediatrics, Yonsei University College of Medicine, Seoul, Korea. KELEE616@yuhs.ac
- 2Institute of Allergy, Yonsei University College of Medicine, Seoul, Korea.
- 3Brain Korea 21 Plus Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea.
- 4Biomedical Science Institute, Yonsei University College of Medicine, Seoul, Korea.
- 5Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2262393
- DOI: http://doi.org/10.4168/aard.2014.2.4.259
Abstract
- PURPOSE
Cow's milk protein is one of the most common and strongest food allergen. We investigated the effects of heat treatment on the distribution and antigenicities of major allergens from cow's milk. We also compared the protein distribution and antigenicities among cow's milk formula and its substitutes.
METHODS
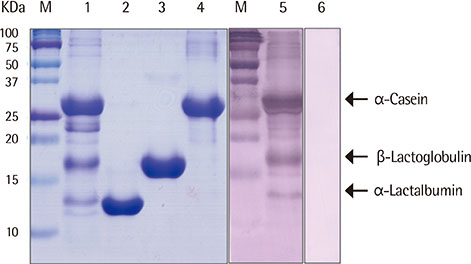
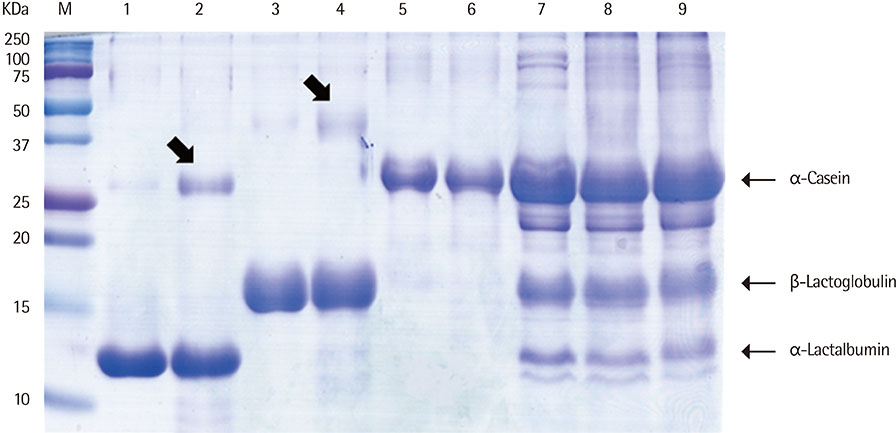
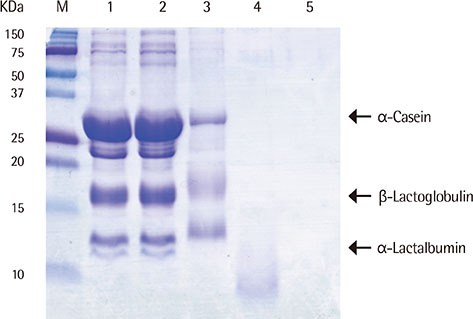
We heated alpha-casen, beta-lactoglobulin (BLG), alpha-lactalbumin (ALA), and crude extract of cow's milk in 100degrees C boiling water for 1 hour. We prepared crude extracts from cow's milk formula, partially hydrolyzed milk formula (pHF) and extensively hydrolyzed milk formula (eHF). The protein compositions of all the samples were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The antigenicities were determined by IgE immunoblotting with pooled serum collected from 11 patients with milk allergy.
RESULTS
After heating, no significant alteration was found in casein, and the aggregates of ALA and BLG were detected with molecular weights of about 30 and 45 kDa, respectively. The antigenicities of newly detected aggregates were increased. The new aggregates of BLG with increased antigenicities were also found in heated milk total protein. Major milk allergens were not found in pHF, and residual components with a molecular weight below 10 KDa did not show IgE-binding activity. We failed to observe the residual components and antigenicities of eHF.
CONCLUSION
Changes in protein distribution and antigenicity of milk total protein induced by heat treatment may not be significantly different from those of each major allergen. The residual components of pHF could have little IgE-binding capacity, and there may be few or no antigenic components in eHF.
Keyword
MeSH Terms
-
Allergens
Caseins
Complex Mixtures
Electrophoresis
Heating
Hot Temperature*
Humans
Hydrolysis*
Immunoblotting
Immunoglobulin E
Lactalbumin
Lactoglobulins
Milk
Milk Hypersensitivity
Milk Proteins*
Molecular Weight
Sodium
Water
Allergens
Caseins
Complex Mixtures
Immunoglobulin E
Lactalbumin
Lactoglobulins
Milk Proteins
Sodium
Water
Figure
Cited by 1 articles
-
The antigenicities of heat treated and hydrolyzed cow's milk protein
So-Yeon Lee
Allergy Asthma Respir Dis. 2014;2(4):227-228. doi: 10.4168/aard.2014.2.4.227.
Reference
-
1. Savilahti E, Kuitunen M. Allergenicity of cow milk proteins. J Pediatr. 1992; 121(5 Pt 2):S12–S20.
Article2. Høst A, Halken S, Jacobsen HP, Christensen AE, Herskind AM, Plesner K. Clinical course of cow's milk protein allergy/intolerance and atopic diseases in childhood. Pediatr Allergy Immunol. 2002; 13:Suppl 15. 23–28.
Article3. Venter C, Pereira B, Grundy J, Clayton CB, Roberts G, Higgins B, et al. Incidence of parentally reported and clinically diagnosed food hypersensitivity in the first year of life. J Allergy Clin Immunol. 2006; 117:1118–1124.
Article4. Hill DJ, Firer MA, Ball G, Hosking CS. Natural history of cows' milk allergy in children: immunological outcome over 2 years. Clin Exp Allergy. 1993; 23:124–131.
Article5. Skripak JM, Matsui EC, Mudd K, Wood RA. The natural history of IgE-mediated cow's milk allergy. J Allergy Clin Immunol. 2007; 120:1172–1177.
Article6. Saarinen KM, Pelkonen AS, Makela MJ, Savilahti E. Clinical course and prognosis of cow's milk allergy are dependent on milk-specific IgE status. J Allergy Clin Immunol. 2005; 116:869–875.
Article7. Wood RA, Sicherer SH, Vickery BP, Jones SM, Liu AH, Fleischer DM, et al. The natural history of milk allergy in an observational cohort. J Allergy Clin Immunol. 2013; 131:805–812.
Article8. Sackesen C, Assaad A, Baena-Cagnani C, Ebisawa M, Fiocchi A, Heine RG, et al. Cow's milk allergy as a global challenge. Curr Opin Allergy Clin Immunol. 2011; 11:243–248.
Article9. Gjesing B, Osterballe O, Schwartz B, Wahn U, Lowenstein H. Allergen-specific IgE antibodies against antigenic components in cow milk and milk substitutes. Allergy. 1986; 41:51–56.
Article10. Sicherer SH, Sampson HA. Cow's milk protein-specific IgE concentrations in two age groups of milk-allergic children and in children achieving clinical tolerance. Clin Exp Allergy. 1999; 29:507–512.
Article11. Bu G, Luo Y, Chen F, Liu K, Zhu T. Milk processing as a tool to reduce cow's milk allergenicity: a mini-review. Dairy Sci Technol. 2013; 93:211–223.
Article12. Kilshaw PJ, Heppell LM, Ford JE. Effects of heat treatment of cow's milk and whey on the nutritional quality and antigenic properties. Arch Dis Child. 1982; 57:842–847.
Article13. Wal JM. Bovine milk allergenicity. Ann Allergy Asthma Immunol. 2004; 93:5 Suppl 3. S2–S11.
Article14. Davis PJ, Smales CM, James DC. How can thermal processing modify the antigenicity of proteins? Allergy. 2001; 56:Suppl 67. 56–60.
Article15. Singh B, Lee KC, Fraga E, Wilkinson A, Wong M, Barton MA. Minimum peptide sequences necessary for priming and triggering of humoral and cell-mediated immune responses in mice: use of synthetic peptide antigens of defined structure. J Immunol. 1980; 124:1336–1343.16. Chung CS, Yamini S, Trumbo PR. FDA's health claim review: whey-protein partially hydrolyzed infant formula and atopic dermatitis. Pediatrics. 2012; 130:e408–e414.
Article17. Isolauri E, Sütas Y, Makinen-Kiljunen S, Oja SS, Isosomppi R, Turjanmaa K. Efficacy and safety of hydrolyzed cow milk and amino acid-derived formulas in infants with cow milk allergy. J Pediatr. 1995; 127:550–557.
Article18. de Boissieu D, Matarazzo P, Dupont C. Allergy to extensively hydrolyzed cow milk proteins in infants: identification and treatment with an amino acid-based formula. J Pediatr. 1997; 131:744–747.
Article19. de Boissieu D, Dupont C. Allergy to extensively hydrolyzed cow's milk proteins in infants: safety and duration of amino acid-based formula. J Pediatr. 2002; 141:271–273.
Article20. Goldman AS, Anderson DW Jr, Sellers WA, Saperstein S, Kniker WT, Halpern SR. Milk allergy. I: oral challenge with milk and isolated milk proteins in allergic children. Pediatrics. 1963; 32:425–443.21. Huang F, Nowak-Wegrzyn A. Extensively heated milk and egg as oral immunotherapy. Curr Opin Allergy Clin Immunol. 2012; 12:283–292.
Article22. Maleki SJ, Hurlburt BK. Structural and functional alterations in major peanut allergens caused by thermal processing. J AOAC Int. 2004; 87:1475–1479.23. Gruber P, Becker WM, Hofmann T. Influence of the maillard reaction on the allergenicity of rAra h 2, a recombinant major allergen from peanut (Arachis hypogaea), its major epitopes, and peanut agglutinin. J Agric Food Chem. 2005; 53:2289–2296.
Article24. Kim TH, Jung HH, Kim ES, Park JY, Kim KW, Sohn MH, et al. A case of rice allergy caused by thin rice porridge during the weaning period. Pediatr Allergy Respir Dis. 2007; 17:149–154.25. Docena GH, Fernandez R, Chirdo FG, Fossati CA. Identification of casein as the major allergenic and antigenic protein of cow's milk. Allergy. 1996; 51:412–416.
Article26. Caubet JC, Nowak-Wegrzyn A, Moshier E, Godbold J, Wang J, Sampson HA. Tility of casein-specific IgE levels in predicting reactivity to baked milk. J Allergy Clin Immunol. 2013; 131:222–224.e1-4.27. Nowak-Wegrzyn A, Bloom KA, Sicherer SH, Shreffler WG, Noone S, Wanich N, et al. Tolerance to extensively heated milk in children with cow's milk allergy. J Allergy Clin Immunol. 2008; 122:342–347. 347.e1–347.e2.
Article28. Baldo B. Milk allergies. Aust J Dairy Technol. 1984; 39:120–128.29. Ehn BM, Ekstrand B, Bengtsson U, Ahlstedt S. Modification of IgE binding during heat processing of the cow's milk allergen beta-lactoglobulin. J Agric Food Chem. 2004; 52:1398–1403.
Article30. Kleber N, Hinrichs J. Antigenic response of β-lactoglobulin in thermally treated bovine skim milk and sweet whey. Milchwissenschaft. 2007; 62:121–124.31. Bu G, Luo Y, Zheng Z, Zheng H. Effect of heat treatment on the antigenicity of bovine α-lactalbumin and β-lactoglobulin in whey protein isolate. Food Agric Immunol. 2009; 20:195–206.
Article32. Anema SG, Li Y. Association of denatured whey proteins with casein micelles in heated reconstituted skim milk and its effect on casein micelle size. J Dairy Res. 2003; 70:73–83.
Article33. Corredig M, Dalgleish DG. The mechanisms of the heat-induced interaction of whey proteins with casein micelles in milk. Int Dairy J. 1999; 9:233–236.
Article34. Schmidt DG, Meijer RJ, Slangen CJ, van Beresteijn EC. Raising the pH of the pepsin-catalysed hydrolysis of bovine whey proteins increases the antigenicity of the hydrolysates. Clin Exp Allergy. 1995; 25:1007–1017.
Article35. van Esch BC, Knipping K, Jeurink P, van der Heide S, Dubois AE, Willemsen LE, et al. In vivo and in vitro evaluation of the residual allergenicity of partially hydrolysed infant formulas. Toxicol Lett. 2011; 201:264–269.
Article36. Docena G, Rozenfeld P, Fernandez R, Fossati CA. Evaluation of the residual antigenicity and allergenicity of cow's milk substitutes by in vitro tests. Allergy. 2002; 57:83–91.
Article37. Restani P, Velona T, Plebani A, Ugazio AG, Poiesi C, Muraro A, et al. Evaluation by SDS-PAGE and immunoblotting of residual antigenicity in hydrolysed protein formulas. Clin Exp Allergy. 1995; 25:651–658.
Article