Allergy Asthma Immunol Res.
2010 Oct;2(4):228-234. 10.4168/aair.2010.2.4.228.
Immunomodulators for Asthma
- Affiliations
-
- 1Division of Allergy & Immunology, Department of Medicine, Creighton University, Omaha, NE, USA. tbcasale@creighton.edu
- KMID: 2260383
- DOI: http://doi.org/10.4168/aair.2010.2.4.228
Abstract
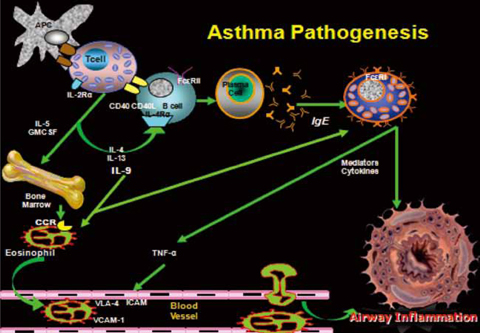
- New information regarding the molecular mechanisms of allergic disorders has led to a variety of novel therapeutic approaches. This article briefly reviews the pathogenesis of asthma and allergic diseases, discusses the rationale behind using immunomodulators in these diseases; and examines the therapeutic effects of immunomodulators on allergic diseases. There are a number of immunomodulators that have been developed for the treatment of allergic disorders. Some have looked very promising in pre-clinical trials, but have not shown significant benefits in human clinical trials thus indicating the disparity between mouse models and human asthma. This review focuses on immunomodulators that are in human clinical trials and not molecules in pre-clinical development.
Keyword
Figure
Reference
-
1. Malmstrom K, Rodriguez-Gomez G, Guerra J, Villaran C, Pineiro A, Wei LX, Seidenberg BC, Reiss TF. Montelukast/Beclomethasone Study Group. Oral montelukast, inhaled beclomethasone, and placebo for chronic asthma. A randomized, controlled trial. Ann Intern Med. 1999. 130:487–495.2. Tantisira KG, Lake S, Silverman ES, Palmer LJ, Lazarus R, Silverman EK, Liggett SB, Gelfand EW, Rosenwasser LJ, Richter B, Israel E, Wechsler M, Gabriel S, Altshuler D, Lander E, Drazen J, Weiss ST. Corticosteroid pharmacogenetics: association of sequence variants in CRHR1 with improved lung function in asthmatics treated with inhaled corticosteroids. Hum Mol Genet. 2004. 13:1353–1359.3. Busse WW, Israel E, Nelson HS, Baker JW, Charous BL, Young DY, Vexler V, Shames RS. Daclizumab improves asthma control in patients with moderate to severe persistent asthma: a randomized, controlled trial. Am J Respir Crit Care Med. 2008. 178:1002–1008.4. Salapatek AM, Patel P, Schulze J, Fischer von Weikersthal-Drachenberg KJ, Zielen S. Comparison of outcomes following ultra short course specific immunotherapy (uSCIT) in juvenile and adult patients with asthma. Proceedings of the XXVIIth Congress of the European Academy of Allergology and Clinical Immunology (EAACI). 2008. Jun 7-11; Barcelona, Spain.5. Creticos PS, Schroeder JT, Hamilton RG, Balcer-Whaley SL, Khattignavong AP, Lindblad R, Li H, Coffman R, Seyfert V, Eiden JJ, Broide D. Immunotherapy with a ragweed-toll-like receptor 9 agonist vaccine for allergic rhinitis. N Engl J Med. 2006. 355:1445–1455.6. Senti G, Johansen P, Haug S, Bull C, Gottschaller C, Müller P, Pfister T, Maurer P, Bachmann MF, Graf N, Kündig TM. Use of A-type CpG oligodeoxynucleotides as an adjuvant in allergen-specific immunotherapy in humans: a phase I/IIa clinical trial. Clin Exp Allergy. 2009. 39:562–570.7. Blaziene A. CYT003-QbG10, a novel allergen-independent immunotherapy, shown to be safe and efficacious in placebo-controlled phase II study. Proceedings of the American College of Allergy, Asthma & Immunology Annual Scientific Meeting. 2008. Nov 6-11; Seattle, USA.8. Belvisi MG, Hele DJ. Peroxisome proliferator-activated receptors as novel targets in lung disease. Chest. 2008. 134:152–157.9. Corren J, Busse W, Meltzer E, Mansfield L, Bensch G, Chon Y, Dunn M, Weng H, Lin S. Efficacy and safety of AMG 317, an IL-4Ra antagonist, in atopic asthmatic subjects: a randomized, double-blind, placebo-controlled study. J Allergy Clin Immunol. 2009. 123:732.10. Wenzel S, Wilbraham D, Fuller R, Getz EB, Longphre M. Effect of an interleukin-4 variant on late phase asthmatic response to allergen challenge in asthmatic patients: results of two phase 2a studies. Lancet. 2007. 370:1422–1431.11. Ishiura Y, Fujimura M, Yamamoto H, Nobata K, Ishiguro T, Ogawa H, Myou S. Effect of an orally active Th2 cytokine inhibitor, suplatast on "atopic cough" tosilate. Arzneimittelforschung. 2008. 58:297–302.12. Agrawal DK, Cheng G, Kim MJ, Kiniwa M. Interaction of suplatast tosilate (IPD) with chloride channels in human blood eosinophils: a potential mechanism underlying its anti-allergic and anti-asthmatic effects. Clin Exp Allergy. 2008. 38:305–312.13. Tanaka A, Minoguchi K, Samson KT, Oda N, Yokoe T, Tazaki T, Yamamoto Y, Yamamoto M, Ohta S, Adachi M. Inhibitory effects of suplatast tosilate on the differentiation and function of monocyte-derived dendritic cells from patients with asthma. Clin Exp Allergy. 2007. 37:1083–1089.14. Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, Marshall RP, Bradding P, Green RH, Wardlaw AJ, Pavord ID. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009. 360:973–984.15. Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, Hargreave FE, O'Byrne PM. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009. 360:985–993.16. Gauvreau GM, Boulet LP, Cockcroft DW, Baatjes A, Cote J, Deschesnes F, Davis B, Strinich T, Howie K, Duong M, Watson RM, Renzi PM, O'Byrne PM. Antisense therapy against CCR3 and the common beta chain attenuates allergen-induced eosinophilic responses. Am J Respir Crit Care Med. 2008. 177:952–958.17. Wenzel SE, Barnes PJ, Bleecker ER, Bousquet J, Busse W, Dahlen SE, Holgate ST, Meyers DA, Rabe KF, Antczak A, Baker J, Horvath I, Mark Z, Bernstein D, Kerwin E, Schlenker-Herceg R, Lo KH, Watt R, Barnathan ES, Chanez P. A randomized, double-blind, placebo-controlled study of tumor necrosis factor-alpha blockade in severe persistent asthma. Am J Respir Crit Care Med. 2009. 179:549–558.18. Meltzer EO, Berkowitz RB, Grossbard EB. An intranasal Syk-kinase inhibitor (R112) improves the symptoms of seasonal allergic rhinitis in a park environment. J Allergy Clin Immunol. 2005. 115:791–796.19. Rigel announces initiation of phase 1 clinical trial of R343 for allergic asthma by its partner Pfizer [Internt]. Rigel Pharmaceuticals, Inc. cited 2009 Jun 12. Available from: http://ir.rigel.com/phoenix.zhtml?c=120936&p=irol-newsArticle&ID=1084738.20. Casale TB, Condemi J, LaForce C, Nayak A, Rowe M, Watrous M, McAlary M, Fowler-Taylor A, Racine A, Gupta N, Fick R, Della Cioppa G. Effect of omalizumab on symptoms of seasonal allergic rhinitis: a randomized controlled trial. JAMA. 2001. 286:2956–2967.21. Casale TB, Stokes J. Anti-IgE therapy: clinical utility beyond asthma. J Allergy Clin Immunol. 2009. 123:770–771.e1.22. Limb SL, Starke PR, Lee CE, Chowdhury BA. Delayed onset and protracted progression of anaphylaxis after omalizumab administration in patients with asthma. J Allergy Clin Immunol. 2007. 120:1378–1381.23. Cox L, Platts-Mills TA, Finegold I, Schwartz LB, Simons FE, Wallace DV. American Academy of Allergy, Asthma & Immunology/American College of Allergy, Asthma and Immunology Joint Task Force Report on omalizumab-associated anaphylaxis. J Allergy Clin Immunol. 2007. 120:1373–1377.24. CRTH2 antagonist [Internet]. Actelion Pharmaceuticals Ltd. cited 2010 Feb 24. Available from: http://www.actelion.com/en/scientists/development-pipeline/phase-2/crth2-antagonist.page.25. Casale TB, Stokes JR. Immunomodulators for allergic respiratory disorders. J Allergy Clin Immunol. 2008. 121:288–296. quiz 97-8.