Allergy Asthma Immunol Res.
2013 Mar;5(2):113-115. 10.4168/aair.2013.5.2.113.
A Case of Levofloxacin-Induced Anaphylaxis With Elevated Serum Tryptase Levels
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea. sanghakim@yonsei.ac.kr
- KMID: 2260348
- DOI: http://doi.org/10.4168/aair.2013.5.2.113
Abstract
- Levofloxacin, a fluoroquinolone and L-isomer of the racemate ofloxacin, has been approved for the treatment of acute and chronic bacterial infections. Gastrointestinal complaints are the most frequently reported adverse drug reactions to fluoroquinolones. Other adverse events include headache, dizziness, increased liver enzyme levels, photosensitivity, tachycardia, QT prolongation, and eruptions. Anaphylaxis has been documented as a rare adverse drug reaction to levofloxacin; however, diagnostic tests are needed to evaluate whether these reactions are the result of levofloxacin treatment. While the results of skin tests are considered unreliable due to false-positive responses, the oral provocation test is currently considered to be the most reliable test. Tryptase, a neutral protease, is the dominant protein component of secretory granules in human mast cells, and an increased serum concentration of tryptase is a highly sensitive indicator of anaphylaxis. Herein, we report a case of levofloxacin-induced anaphylaxis in which the patient exhibited elevated serum tryptase levels and a positive oral levofloxacin challenge test result. As anaphylaxis is potentially life-threatening, the administration of fluoroquinolones to patients who have experienced a prior reaction to this type of agent should be avoided.
Keyword
MeSH Terms
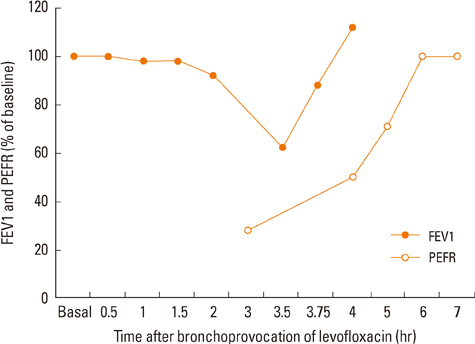
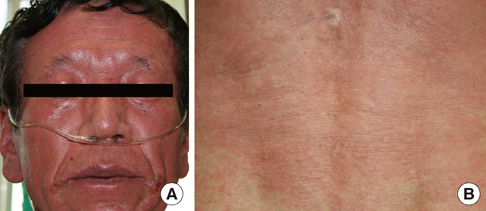
Figure
Cited by 1 articles
-
Overview of anaphylaxis in Korea: diagnosis and management
Gwang Cheon Jang, Yoon-Seok Chang, Sun Hee Choi, Woo-Jung Song, Soo-Young Lee, Hae-Sim Park, Hye-Ryun Kang, Yeong-Min Ye, Hyun-Jung Jin, Mi Yong Shin, Soo-Jin Lee, Hye One Kim, Jihyun Kim, Jae-Woo Jung, Hee-Bom Moon, Youngmin Ahn
Allergy Asthma Respir Dis. 2013;1(3):181-196. doi: 10.4168/aard.2013.1.3.181.
Reference
-
1. Croom KF, Goa KL. Levofloxacin: a review of its use in the treatment of bacterial infections in the United States. Drugs. 2003. 63:2769–2802.2. Lipsky BA, Baker CA. Fluoroquinolone toxicity profiles: a review focusing on newer agents. Clin Infect Dis. 1999. 28:352–364.3. Smythe MA, Cappelletty DM. Anaphylactoid reaction to levofloxacin. Pharmacotherapy. 2000. 20:1520–1523.4. Takahama H, Tsutsumi Y, Kubota Y. Anaphylaxis due to levofloxacin. Int J Dermatol. 2005. 44:789–790.5. Sachs B, Riegel S, Seebeck J, Beier R, Schichler D, Barger A, Merk HF, Erdmann S. Fluoroquinolone-associated anaphylaxis in spontaneous adverse drug reaction reports in Germany: differences in reporting rates between individual fluoroquinolones and occurrence after first-ever use. Drug Saf. 2006. 29:1087–1100.6. Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, Motala C, Ortega Martell JA, Platts-Mills TA, Ring J, Thien F, Van Cauwenberge P, Williams HC. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol. 2004. 113:832–836.7. Aranda A, Mayorga C, Ariza A, Doña I, Rosado A, Blanca-Lopez N, Andreu I, Torres MJ. In vitro evaluation of IgE-mediated hypersensitivity reactions to quinolones. Allergy. 2011. 66:247–254.8. Manfredi M, Severino M, Testi S, Macchia D, Ermini G, Pichler WJ, Campi P. Detection of specific IgE to quinolones. J Allergy Clin Immunol. 2004. 113:155–160.9. Borchers AT, Naguwa SM, Keen CL, Gershwin ME. The diagnosis and management of anaphylaxis. Compr Ther. 2004. 30:111–120.10. Kelesidis T, Fleisher J, Tsiodras S. Anaphylactoid reaction considered ciprofloxacin related: a case report and literature review. Clin Ther. 2010. 32:515–526.11. Campi P, Pichler WJ. Quinolone hypersensitivity. Curr Opin Allergy Clin Immunol. 2003. 3:275–281.12. Schwartz LB, Metcalfe DD, Miller JS, Earl H, Sullivan T. Tryptase levels as an indicator of mast-cell activation in systemic anaphylaxis and mastocytosis. N Engl J Med. 1987. 316:1622–1626.13. Brown SG, Blackman KE, Heddle RJ. Can serum mast cell tryptase help diagnose anaphylaxis? Emerg Med Australas. 2004. 16:120–124.14. Payne V, Kam PC. Mast cell tryptase: a review of its physiology and clinical significance. Anaesthesia. 2004. 59:695–703.15. Shiohara T, Kano Y. A complex interaction between drug allergy and viral infection. Clin Rev Allergy Immunol. 2007. 33:124–133.16. Pirmohamed M, Park BK. HIV and drug allergy. Curr Opin Allergy Clin Immunol. 2001. 1:311–316.17. Hashimoto K, Yasukawa M, Tohyama M. Human herpesvirus 6 and drug allergy. Curr Opin Allergy Clin Immunol. 2003. 3:255–260.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Utility of Serum Tryptase in the Diagnosis of Food-Induced Anaphylaxis
- Elevated level of serum tryptase in a patient with exercise-inuced anaphylaxis
- A Case of Diclofenac-Induced Anaphylaxis with Elevated Serum Tryptase
- Causes and Diagnostic Usefulness of Tryptase Measurements for Anaphylaxis in a Korean Tertiary Care General Hospital
- Anaphylactic Shock by Hemocoagulase with Increased Concentration of Mast Cell Tryptase: A case report