Allergy Asthma Immunol Res.
2013 Nov;5(6):383-388. 10.4168/aair.2013.5.6.383.
Threshold for Positivity and Optimal Dipyrone Concentration in Flow Cytometry-Assisted Basophil Activation Test
- Affiliations
-
- 1Department of Anaesthesia and Intensive Care, University of Medicine and Pharmacy "Iuliu Hatieganu" Cluj-Napoca, Romania. hagaunatalia@gmail.com
- 2Universite Paris-Diderot, Hopitaux Universitaires Paris Nord Val de Seine, Departement d'Anesthesie Reanimation Chirurgicale, Hopital Bichat-Claude Bernard, Paris, France.
- KMID: 2260282
- DOI: http://doi.org/10.4168/aair.2013.5.6.383
Abstract
- PURPOSE
Basophil activation occurs both in patients with immediate hypersensitivity reactions to anti-inflammatory drugs and in healthy controls in a dose-dependent manner. Our aims were to define the optimal basophil activation test (BAT) concentration and the threshold for BAT positivity for dipyrone.
METHODS
From 45 patients with a positive history of an immediate hypersensitivity reaction to dipyrone, we found 20 patients with dipyrone-induced anaphylaxis demonstrating positive skin tests. All selected patients, as well as 10 healthy controls, were tested in vivo and in vitro. BAT was performed using Flow 2CAST technique with three low dipyrone concentrations: 25 microg/mL (c1), 2.5 microg/mL (c2) and 0.25 microg/mL (c3). The threshold for BAT positivity was established using receiver operating characteristics (ROC) curve analysis.
RESULTS
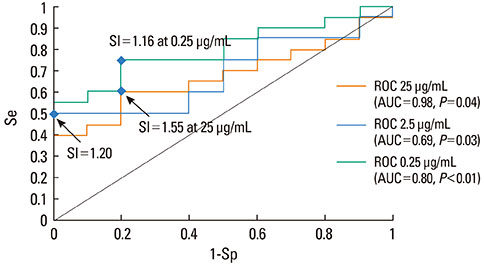
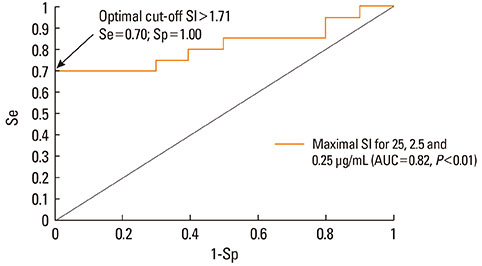
Using ROC curve analysis the highest area under curve, 0.79 (0.63-0.95) (P<0.01), was found for c3. When the highest stimulation indexes from the three concentrations for each patient were used, ROC curve analysis revealed an area under curve of 0.81 (0.65-0.96) (P<0.01), sensitivity and specificity were 0.70 and 1 and the optimal threshold value for BAT positivity was 1.71. Thirteen patients had a positive BAT for at least one of the tested dipyrone concentrations. All healthy controls presented negative BAT.
CONCLUSIONS
BAT might be a useful technique to diagnose dipyrone allergy, provided all three low dipyrone concentrations are used together. With an assay-specific threshold of 1.71, ROC curve analysis yields 70% sensitivity and 100% specificity.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Recent applications of basophil activation tests in the diagnosis of drug hypersensitivity
Woo-Jung Song, Yoon-Seok Chang
Asia Pac Allergy. 2013;3(4):266-280. doi: 10.5415/apallergy.2013.3.4.266.
Reference
-
1. Levy M. Hypersensitivity to pyrazolones. Thorax. 2000; 55:Suppl 2. S72–S74.2. Braga TB, Pfaffenbach G, Weiss DP, Barros MB, Bergsten-Mendes G. Point prevalence of drug prescriptions for elderly and non-elderly inpatients in a teaching hospital. Sao Paulo Med J. 2004; 122:48–52.3. Grundmann U, Wörnle C, Biedler A, Kreuer S, Wrobel M, Wilhelm W. The efficacy of the non-opioid analgesics parecoxib, paracetamol and metamizol for postoperative pain relief after lumbar microdiscectomy. Anesth Analg. 2006; 103:217–222.4. Kampe S, Warm M, Landwehr S, Dagtekin O, Haussmann S, Paul M, Pilgram B, Kiencke P. Clinical equivalence of IV paracetamol compared to IV dipyrone for postoperative analgesia after surgery for breast cancer. Curr Med Res Opin. 2006; 22:1949–1954.5. Nauck F, Ostgathe C, Klaschik E, Bausewein C, Fuchs M, Lindena G, Neuwöhner K, Schulenberg D, Radbruch L. Working Group on the Core Documentation for Palliative Care Units in Germany. Drugs in palliative care: results from a representative survey in Germany. Palliat Med. 2004; 18:100–107.6. Lefterova A, Getov I. Study on consumers' preferences and habits for over-the-counter analgesics use. Cent Eur J Public Health. 2004; 12:43–45.7. Campi P, Manfredi M, Severino M. IgE-mediated allergy to pyrazolones, quinolones and other non-β-lactam antibiotics. In : Pichler WJ, editor. Drug hypersensitivity. Basel: Karger;2007. p. 216–232.8. Gómez E, Blanca-Lopez N, Torres MJ, Requena G, Rondon C, Canto G, Blanca M, Mayorga C. Immunoglobulin E-mediated immediate allergic reactions to dipyrone: value of basophil activation test in the identification of patients. Clin Exp Allergy. 2009; 39:1217–1224.9. Gamboa PM, Sanz ML, Caballero MR, Antépara I, Urrutia I, Jáuregui I, González G, Diéguez I, De Weck AL. Use of CD63 expression as a marker of in vitro basophil activation and leukotriene determination in metamizol allergic patients. Allergy. 2003; 58:312–317.10. Hausmann OV, Gentinetta T, Bridts CH, Ebo DG. The basophil activation test in immediate-type drug allergy. Immunol Allergy Clin North Am. 2009; 29:555–566.11. Ebo DG, Bridts CH, Hagendorens MM, Mertens CH, De Clerck LS, Stevens WJ. Flow-assisted diagnostic management of anaphylaxis from rocuronium bromide. Allergy. 2006; 61:935–939.12. De Week AL, Sanz ML, Gamboa PM, Aberer W, Sturm G, Bilo MB, Montroni M, Blanca M, Torres MJ, Mayorga L, Campi P, Manfredi M, Drouet M, Sainte-Laudy J, Romano A, Merk H, Weber JM, Jermann TM. ENDA (European Network for Drug Allergy). Diagnosis of immediate-type beta-lactam allergy in vitro by flow-cytometric basophil activation test and sulfidoleukotriene production: a multicenter study. J Investig Allergol Clin Immunol. 2009; 19:91–109.13. Mayorga C, Sanz ML, Gamboa PM, García BE, Caballero MT, García JM, Labrador M, Lahoz C, Longo Areso N, López Hoyos M, Martínez Quesada J, Monteseirín FJ. Immunology Committee of the Spanish Society of Allergology and Clinical Immunology of the SEAIC. In vitro diagnosis of immediate allergic reactions to drugs: an update. J Investig Allergol Clin Immunol. 2010; 20:103–109.14. Gamboa P, Sanz ML, Caballero MR, Urrutia I, Antépara I, Esparza R, de Weck AL. The flow-cytometric determination of basophil activation induced by aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs) is useful for in vitro diagnosis of the NSAID hypersensitivity syndrome. Clin Exp Allergy. 2004; 34:1448–1457.15. De Weck AL, Sanz ML, Gamboa PM, Jermann JM, Kowalski M, Medrala W, Sainte-Laudy J, Schneider MS, Weber JM, Wolanczyk-Medrala A. Nonsteroidal anti-inflammatory drug hypersensitivity syndrome: a multicenter study. II. Basophil activation by nonsteroidal anti-inflammatory drugs and its impact on pathogenesis. J Investig Allergol Clin Immunol. 2010; 20:39–57.16. Gherman-Ionică N, Bologa R, Cocu S, Cristea C, Dîrzu D, Hagău N. Proposal for use of two concentrations for metamizol to intradermal testing. Farmacia. 2011; 59:578–589.17. Mertes PM, Laxenaire MC, Lienhart A, Aberer W, Ring J, Pichler WJ, Demoly P. Working Group for the SFAR. ENDA. EAACI Interest Group on Drug Hypersensitivity. Reducing the risk of anaphylaxis during anaesthesia: guidelines for clinical practice. J Investig Allergol Clin Immunol. 2005; 15:91–101.18. Demoly P, Piette V, Bousquet J. In vivo methods for study of allergy: skin tests, technique and interpretation. In : Adkinson NF, Yunginger JW, Busse WW, Bochner BS, Holgate ST, Simons RE, editors. Middleton's allergy: principles and practice. 6th ed. Philadelphia, PA: Mosby;2003. p. 631–643.19. Bamber D. The area above the ordinal dominance graph and the area below the receiver operating characteristic graph. J Math Psychol. 1975; 12:387–415.20. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982; 143:29–36.21. Zhou XH, Obuchowski NA, McClish DK. Statistical methods in diagnostic medicine. New York: Wiley-Interscience;2002.22. Fischer SS. Anaphylaxis in anaesthesia and critical care. Curr Allergy Clin Immunol. 2007; 20:136–139.23. Mertes PM, Tajima K, Regnier-Kimmoun MA, Lambert M, Iohom G, Guéant-Rodriguez RM, Malinovsky JM. Perioperative anaphylaxis. Med Clin North Am. 2010; 94:761–789.24. Himly M, Jahn-Schmid B, Pittertschatscher K, Bohle B, Grubmayr K, Ferreira F, Ebner H, Ebner C. IgE-mediated immediate-type hypersensitivity to the pyrazolone drug propyphenazone. J Allergy Clin Immunol. 2003; 111:882–888.25. Sanz ML, Gamboa PM, De Weck AL. Cellular tests in the diagnosis of drug hypersensitivity. Curr Pharm Des. 2008; 14:2803–2808.26. Ebo DG, Leysen J, Mayorga C, Rozieres A, Knol EF, Terreehorst I. The in vitro diagnosis of drug allergy: status and perspectives. Allergy. 2011; 66:1275–1286.27. Bleasel KE, Donnan G, Unglik GA. General anesthetic allergy testing. Curr Allergy Asthma Rep. 2009; 9:50–56.28. De Week AL, Sanz ML, Gamboa PM, Aberer W, Bienvenu J, Blanca M, Demoly P, Ebo DG, Mayorga L, Monneret G, Sainte Laudy J. Diagnostic tests based on human basophils: more potentials and perspectives than pitfalls II Technical issues. J Investig Allergol Clin Immunol. 2008; 18:143–155.29. Ebo DG, Bridts CH, Hagendorens MM, Aerts NE, De Clerck LS, Stevens WJ. Basophil activation test by flow cytometry: present and future applications in allergology. Cytometry B Clin Cytom. 2008; 74:201–210.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Basophil Activation Tests Based on CD193 Marker in Dipyrone Allergy
- Fexofenadine-Induced Urticaria
- Flow Cytometry-Assisted Basophil Activation Test as a Safe Diagnostic Tool for Aspirin/NSAID Hypersenstivity
- Recent applications of basophil activation tests in the diagnosis of drug hypersensitivity
- Basophil Markers for Identification and Activation in the Indirect Basophil Activation Test by Flow Cytometry for Diagnosis of Autoimmune Urticaria