Allergy Asthma Immunol Res.
2012 May;4(3):137-142. 10.4168/aair.2012.4.3.137.
Flow Cytometry-Assisted Basophil Activation Test as a Safe Diagnostic Tool for Aspirin/NSAID Hypersenstivity
- Affiliations
-
- 1Department of Internal Medicine, Soon Chun Hyang University Gumi Hospital, Gumi, Korea.
- 2Department of Internal Medicine, Ewha Womans University School of Medicine, Seoul, Korea. yjcho@ewha.ac.kr
- KMID: 2167065
- DOI: http://doi.org/10.4168/aair.2012.4.3.137
Abstract
- PURPOSE
Aspirin and non-steroidal anti-inflammatory drugs (ASA/NSAIDs) are common causes of drug hypersensitivity. An oral provocation test is the only definitive diagnostic test. This study assessed the reliability of a flow cytometry-assisted basophil activation test (FAST) as a safe diagnostic method for ASA/NSAID-induced hypersensitivity, as its high sensitivity and specificity have been demonstrated for many other drugs.
METHODS
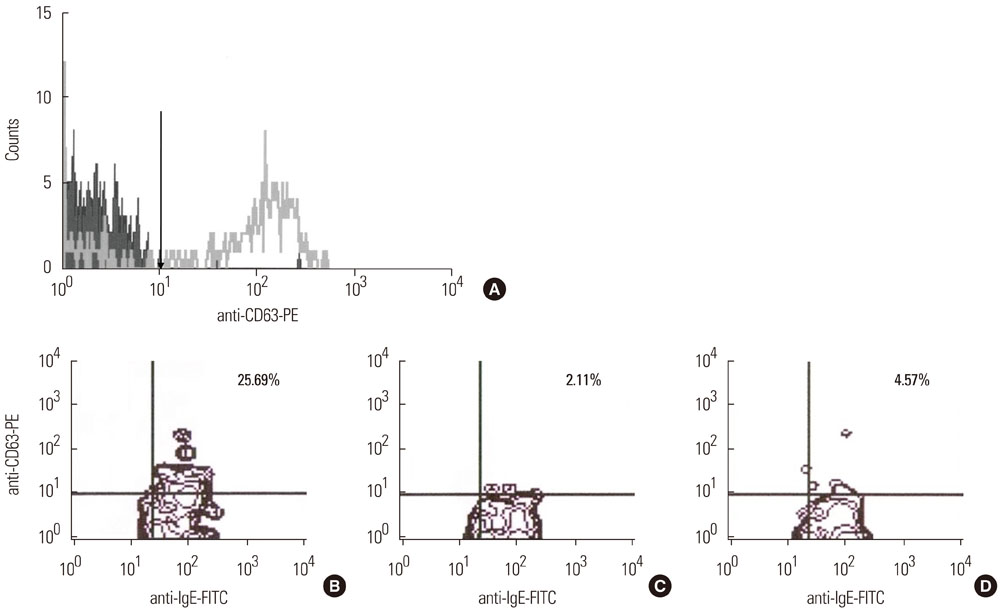
Eighteen patients and 11 controls were enrolled. Using a Flow-CAST kit(R) (Buhlmann Laboratories AG, Schonenbuch, Switzerland), 29 analyses with aspirin, ibuprofen, and diclofenac were performed by flow cytometry to detect double-positive staining of anti-IgE and anti-CD63. The stimulation index was defined as the activated basophil percentage after drug stimulation/basally active basophil percentage. A stimulation index> or =2 and an absolute activated basophil percentage> or =5 were considered positive.
RESULTS
Patients with hypersensitivity to ASA/NSAIDs were predominantly female, and the prevalence of atopy was higher in patients than in controls. A sensitivity of 61%, specificity of 91%, positive predictive value of 92%, and negative predictive value of 59% were achieved.
CONCLUSIONS
FAST is a useful additional method for diagnosis of hypersensitivity reactions to ASA/NSAIDs. Further development is required to increase the sensitivity of the test.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Immunologic Evaluation of Immediate Hypersensitivity to Cefaclor
Hye-Soo Yoo, Seung-Hyun Kim, Hyouk-Soo Kwon, Tae-Bum Kim, Young-Hee Nam, Young-Min Ye, Hae-Sim Park
Yonsei Med J. 2014;55(6):1473-1483. doi: 10.3349/ymj.2014.55.6.1473.Recent applications of basophil activation tests in the diagnosis of drug hypersensitivity
Woo-Jung Song, Yoon-Seok Chang
Asia Pac Allergy. 2013;3(4):266-280. doi: 10.5415/apallergy.2013.3.4.266.
Reference
-
1. Szczeklik A, Stevenson DD. Aspirin-induced asthma: advances in pathogenesis, diagnosis, and management. J Allergy Clin Immunol. 2003. 111:913–921.2. Kasper L, Sladek K, Duplaga M, Bochenek G, Liebhart J, Gladysz U, Malolepszy J, Szczeklik A. Prevalence of asthma with aspirin hypersensitivity in the adult population of Poland. Allergy. 2003. 58:1064–1066.3. Choi JH, Shin YS, Suh CH, Nahm DH, Park HS. The frequency of adverse drug reactions in a tertiary care hospital in Korea. Korean J Med. 2004. 67:290–296.4. Nizankowska-Mogilnicka E, Bochenek G, Mastalerz L, Swierczyńska M, Picado C, Scadding G, Kowalski ML, Setkowicz M, Ring J, Brockow K, Bachert C, Wöhrl S, Dahlén B, Szczeklik A. EAACI/GA2LEN guideline: aspirin provocation tests for diagnosis of aspirin hypersensitivity. Allergy. 2007. 62:1111–1118.5. Palma-Carlos AG, Medina M, Palma-Carlos ML. Skin tests in NSAIDS hypersensitivity. Eur Ann Allergy Clin Immunol. 2006. 38:182–185.6. Asero R. Predictive value of autologous plasma skin test for multiple nonsteroidal anti-inflammatory drug intolerance. Int Arch Allergy Immunol. 2007. 144:226–230.7. Asero R, Tedeschi A, Lorini M. Autoreactivity is highly prevalent in patients with multiple intolerances to NSAIDs. Ann Allergy Asthma Immunol. 2002. 88:468–472.8. Mastalerz L, Setkowicz M, Sanak M, Szczeklik A. Hypersensitivity to aspirin: common eicosanoid alterations in urticaria and asthma. J Allergy Clin Immunol. 2004. 113:771–775.9. Kim SH, Jeong HH, Cho BY, Kim M, Lee HY, Lee J, Wee K, Park HS. Association of four-locus gene interaction with aspirin-intolerant asthma in Korean asthmatics. J Clin Immunol. 2008. 28:336–342.10. Choi JH, Lee KW, Oh HB, Lee KJ, Suh YJ, Park CS, Park HS. HLA association in aspirin-intolerant asthma: DPB1*0301 as a strong marker in a Korean population. J Allergy Clin Immunol. 2004. 113:562–564.11. Kowalski ML, Ptasinska A, Jedrzejczak M, Bienkiewicz B, Cieslak M, Grzegorczyk J, Pawliczak R, Dubuske L. Aspirin-triggered 15-HETE generation in peripheral blood leukocytes is a specific and sensitive Aspirin-Sensitive Patients Identification Test (ASPITest). Allergy. 2005. 60:1139–1145.12. May A, Weber A, Gall H, Kaufmann R, Zollner TM. Means of increasing sensitivity of an in vitro diagnostic test for aspirin intolerance. Clin Exp Allergy. 1999. 29:1402–1411.13. Pierzchalska M, Mastalerz L, Sanak M, Zazula M, Szczeklik A. A moderate and unspecific release of cysteinyl leukotrienes by aspirin from peripheral blood leucocytes precludes its value for aspirin sensitivity testing in asthma. Clin Exp Allergy. 2000. 30:1785–1791.14. Boumiza R, Debard AL, Monneret G. The basophil activation test by flow cytometry: recent developments in clinical studies, standardization and emerging perspectives. Clin Mol Allergy. 2005. 3:9.15. Ebo DG, Bridts CH, Hagendorens MM, Aerts NE, De Clerck LS, Stevens WJ. Basophil activation test by flow cytometry: present and future applications in allergology. Cytometry B Clin Cytom. 2008. 74:201–210.16. Torres MJ, Padial A, Mayorga C, Fernández T, Sanchez-Sabate E, Cornejo-García JA, Antúnez C, Blanca M. The diagnostic interpretation of basophil activation test in immediate allergic reactions to betalactams. Clin Exp Allergy. 2004. 34:1768–1775.17. Monneret G, Benoit Y, Debard AL, Gutowski MC, Topenot I, Bienvenu J. Monitoring of basophil activation using CD63 and CCR3 in allergy to muscle relaxant drugs. Clin Immunol. 2002. 102:192–199.18. Gamboa P, Sanz ML, Caballero MR, Urrutia I, Antépara I, Esparza R, de Weck AL. The flow-cytometric determination of basophil activation induced by aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs) is useful for in vitro diagnosis of the NSAID hypersensitivity syndrome. Clin Exp Allergy. 2004. 34:1448–1457.19. Sanz ML, Gamboa P, de Weck AL. A new combined test with flowcytometric basophil activation and determination of sulfidoleukotrienes is useful for in vitro diagnosis of hypersensitivity to aspirin and other nonsteroidal anti-inflammatory drugs. Int Arch Allergy Immunol. 2005. 136:58–72.20. Sainte-Laudy J, Touraine F, Boumediene A, Bonnaud F, Cogné M. Clinico-biological characteristics of flow cytometry applied to hypersensitivity to NSAIDs. Inflamm Res. 2007. 56:Suppl 1. S63–S64.21. Rodríguez-Trabado A, Cámara-Hijón C, Ramos-Cantariño A, Porcel-Carreño SL, Jiménez-Timón S, Pereira-Navarro G, Hernández-Arbeiza FJ, Fernández-Pereira L. Basophil activation test for the in vitro diagnosis of nonsteroidal anti-inflammatory drug hypersensitivity. Allergy Asthma Proc. 2008. 29:241–249.22. Malbrán A, Yeyati E, Rey GL, Galassi N. Diclofenac induces basophil degranulation without increasing CD63 expression in sensitive patients. Clin Exp Immunol. 2007. 147:99–105.23. Gamboa PM, Sanz ML, Caballero MR, Antépara I, Urrutia I, Jáuregui I, González G, Diéguez I, De Weck AL. Use of CD63 expression as a marker of in vitro basophil activation and leukotriene determination in metamizol allergic patients. Allergy. 2003. 58:312–317.24. Adkinson NF, Yunginger JW, Busse WW, Bochner BS, Holgate ST, Simons FER. Middleton's allergy: principles & practice. 2003. 6th ed. Philadelphia: Mosby;1695–1710.25. McEvoy GK. AHFS drug information. 2006. Wisconsin: American Society of Health-System Pharmacists;2010. 2021. 2038.26. Celik GE, Schroeder JT, Hamilton RG, Saini SS, Adkinson NF. Effect of in vitro aspirin stimulation on basophils in patients with aspirin-exacerbated respiratory disease. Clin Exp Allergy. 2009. 39:1522–1531.27. Bavbek S, Ikincioğullari A, Dursun AB, Guloğlu D, Arikan M, Elhan AH, Misirligil Z. Upregulation of CD63 or CD203c alone or in combination is not sensitive in the diagnosis of nonsteroidal anti-inflammatory drug intolerance. Int Arch Allergy Immunol. 2009. 150:261–270.28. Boumiza R, Monneret G, Forissier MF, Savoye J, Gutowski MC, Powell WS, Bienvenu J. Marked improvement of the basophil activation test by detecting CD203c instead of CD63. Clin Exp Allergy. 2003. 33:259–265.29. Sainte-Laudy J, Belon P. Improvement of flow cytometric analysis of basophil activation inhibition by high histamine dilutions. A novel basophil specific marker: CD 203c. Homeopathy. 2006. 95:3–8.30. Ebo DG, Sainte-Laudy J, Bridts CH, Mertens CH, Hagendorens MM, Schuerwegh AJ, De Clerck LS, Stevens WJ. Flow-assisted allergy diagnosis: current applications and future perspectives. Allergy. 2006. 61:1028–1039.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent applications of basophil activation tests in the diagnosis of drug hypersensitivity
- Fexofenadine-Induced Urticaria
- Basophil Markers for Identification and Activation in the Indirect Basophil Activation Test by Flow Cytometry for Diagnosis of Autoimmune Urticaria
- Flow-Assisted Differential Diagnosis of Hemolytic Anemia with Spherocytosis: A Case Report
- The Basophil Activation Test Is Safe and Useful for Confirming Drug-Induced Anaphylaxis