Korean J Hematol.
2005 Jun;40(2):101-105. 10.5045/kjh.2005.40.2.101.
The Long Term Remission Effect of Rituximab in Two Patients with Autoimmune-associated Cytopenias that were Refractory to Standard Treatments
- Affiliations
-
- 1Division of Hemato-Oncology, Department of Internal Medicine, Our Lady of Mercy Hospital, The Catholic University of Korea College of Medicine, Seoul, Korea. cwhan@unitel.co.kr
- KMID: 2252344
- DOI: http://doi.org/10.5045/kjh.2005.40.2.101
Abstract
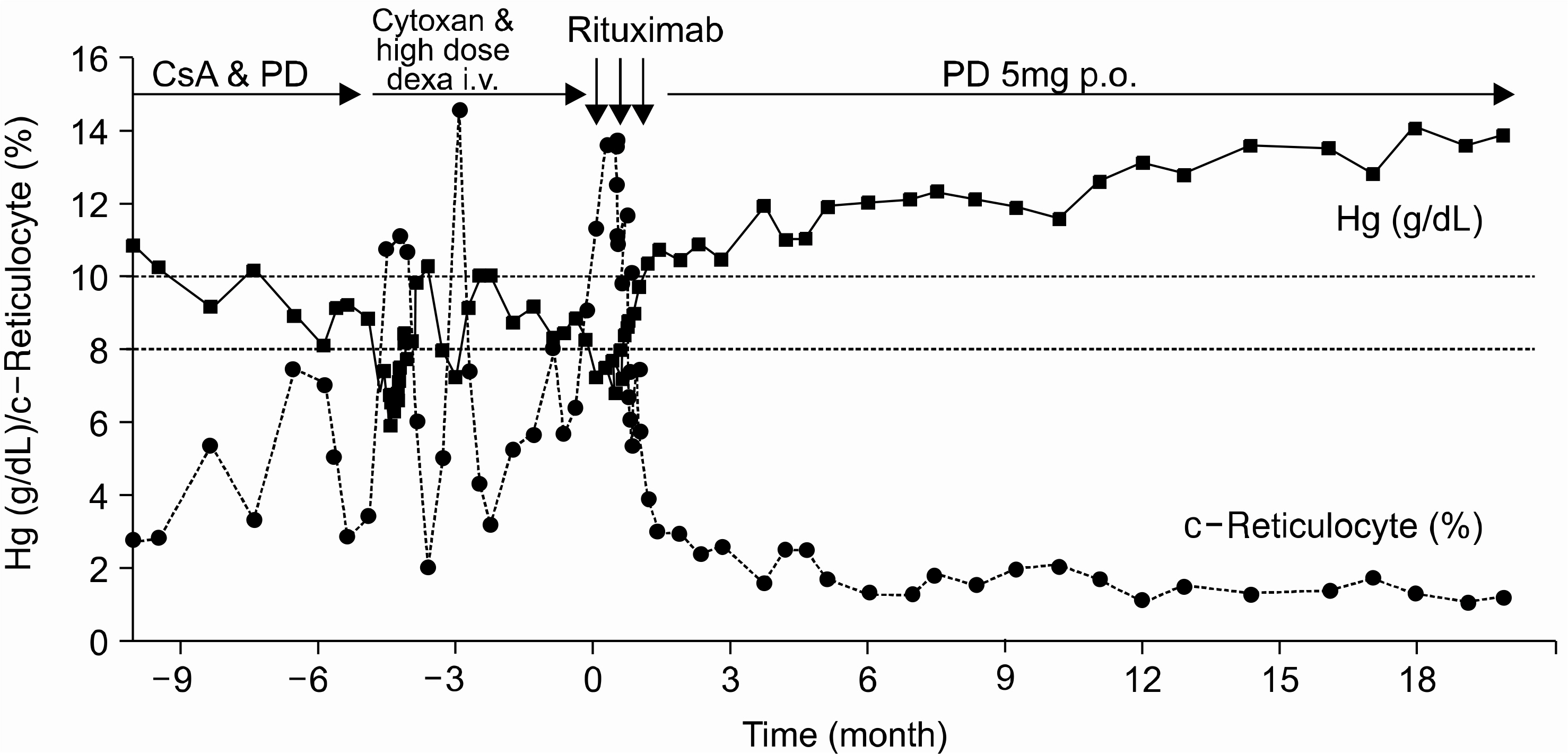
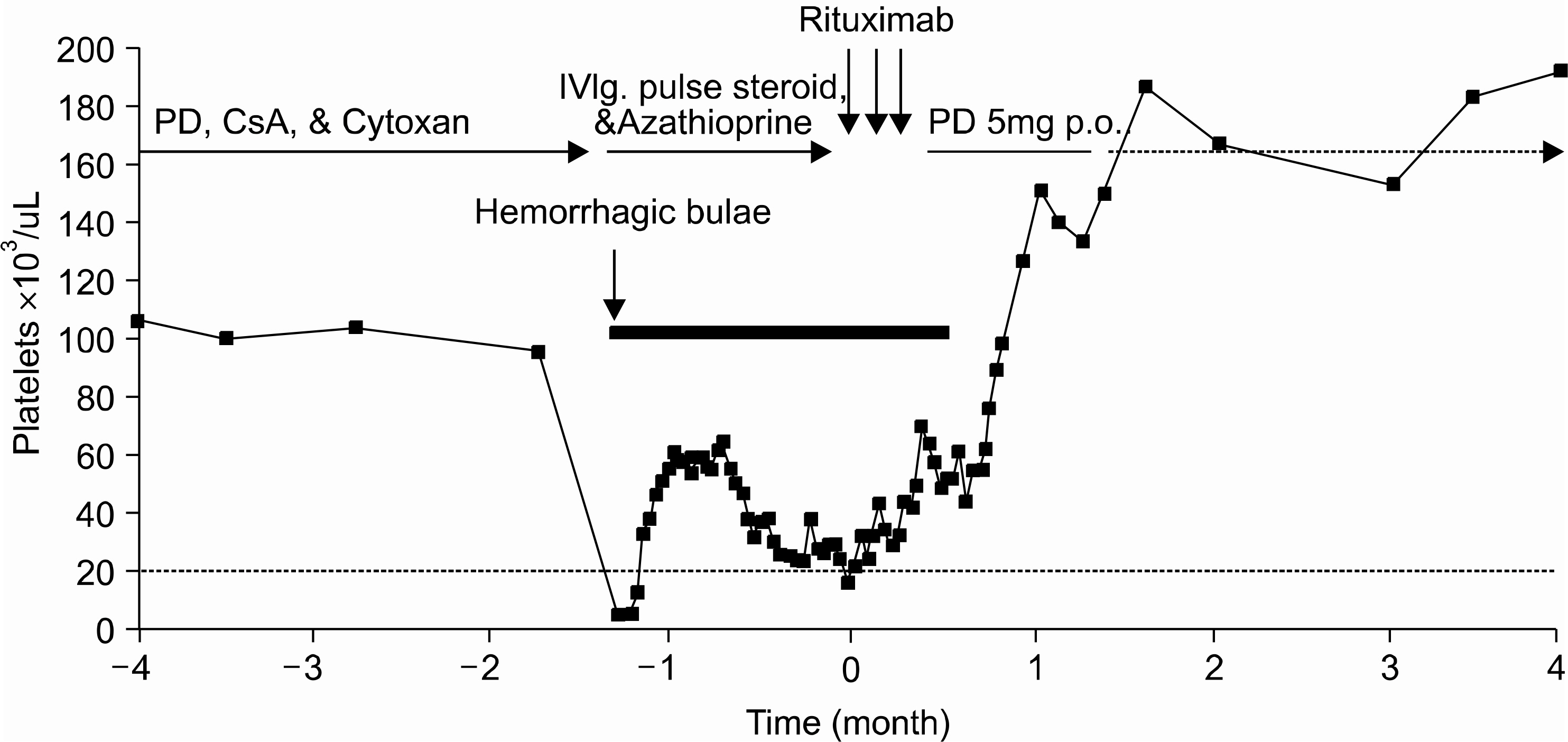
- Rituximab, anti-CD20 chimeric monoclonal antibody directed against the CD20 antigen on B lymphocytes, induces a targeted B lymphocytes depletion in the aim of eradicating autoreactive clones in various autoimmune disorders. Because of its biological properties, it has been used as a treatment option for a variety of autoimmune diseases. We report two complicated patients: a 26-year-old female with steroid induced Cushing's syndrome and avascular necrosis of both femur heads, and a 56-year-old female with multiple spine compression fractures due to osteoporosis, diabetes mellitus and cataracts. They had long lasting, more than 10 years, lupus-associated hemolytic anemia and Evans syndrome, refractory to corticosteroids and immunosuppressive agents. The patients were treated with Rituximab, 375mg/m2 once weekly for 3 consecutive weeks. They showed a remarkable recovery about 5th week and have been free of transfusion after the treatment with Rituximab. Therapy was well tolerated, and no infectious complications occurred. They are still in complete remission at 20 and 4 months following the treatment, respectively. We suggest that Rituximab can be a valuable agent in the management of autoimmune cytopenias refractory to standard treatments.
Keyword
MeSH Terms
-
Adrenal Cortex Hormones
Adult
Anemia, Hemolytic
Antigens, CD20
Autoimmune Diseases
B-Lymphocytes
Cataract
Clone Cells
Cushing Syndrome
Diabetes Mellitus
Female
Femur Head
Fractures, Compression
Humans
Immunosuppressive Agents
Middle Aged
Necrosis
Osteoporosis
Spine
Rituximab
Adrenal Cortex Hormones
Antigens, CD20
Immunosuppressive Agents
Figure
Cited by 1 articles
-
A Case of Autoimmune Hemolytic Anemia Treated with Rituximab in a Child
Ji Hye Lee, Kun Soo Lee
Korean J Hematol. 2006;41(4):321-325. doi: 10.5045/kjh.2006.41.4.321.
Reference
-
1). Yang DH, Lee JJ, Kim YK, et al. The clinical efficacy of R-CHOP chemotherapy in patients with previously untreated diffuse large B-cell lymphoma. Korean J Hematol. 2004; 39:59–65.2). Gibson AD. Rituximab improves rate and duration of chemotherapy-induced remissions in indolent and aggressive Non-Hodgkins lymphoma. Clin Lymphoma. 2004; 5:81–3.
Article3). Dillman RO. Treatment of lowgrade B-cell lymphoma with the monoclonal antibody rituximab. Semin Oncol. 2003; 30:434–47.
Article4). Park US, Choi CB, Kim IS, et al. A case of post-transplantation lymphoproliferative disease developed in renal transplant recipient and treated with rituximab. Korean J Med. 2004; 67:94–9.5). Ganne V, Siddiqi N, Kamaplath B, et al. Humanized anti-CD20 monoclonal antibody (Rituximab) treatment for post-transplant lymphoproliferative disorder. Clin Transplant. 2003; 17:417–22.
Article6). Chambers SA, Isenberg D. Anti-B cell therapy (rituximab) in the treatment of autoimmune diseases. Lupus. 2005; 14:210–4.
Article7). Bonduel M, Zelazko M, Figueroa C, Magaldi G, Rossi J, del Pozo A. Successful treatment of autoimmune hemolytic anemia with rituximab in a child with severe combined immunodeficiency following nonidentical T-cell-depleted bone marrow transplantation. Bone Marrow Transplant. 2005; 35:819–21.
Article8). Raj K, Narayanan S, Augustson B, et al. Rituximab is effective in the management of refractory autoimmune cytopenias occurring after allogeneic stem cell transplantation. Bone Marrow Transplant. 2005; 35:299–301.
Article9). Ten Cate R, Smiers FJ, Bredius RG, et al. Anti-CD20 monoclonal antibody (rituximab) for refractory autoimmune thrombocytopenia in a girl with systemic lupus erythematosus. Rheumatology (Oxford). 2004; 43:244.
Article10). Kornberg AJ, Pestronk A. Antibody-associated polyneuropathy syndromes: principles and treatment. Semin Neurol. 2003; 23:181–90.
Article11). Fry AM, Jones LA, Kruisbeek AM, Matis LA. Thymic requirement for clonal deletion during T cell development. Science. 1989; 24:1044–6.
Article12). Williams JA, Sharrow SO, Adams AJ, Hodes RJ. CD40 ligand functions non-cell autonomously to promote deletion of self-reactive thymocytes. J Immunol. 2002; 168:2759–65.
Article13). Levin MC, Lee SM, Kalume F, et al. Autoimmunity due to molecular mimicry as a cause of neurological disease. Nat Med. 2002; 8:509–13.
Article14). Wautier JL, Rouger P. Drug-induced hemolytic anemia. Transfus Clin Biol. 2001; 8:377–80.15). Gasch AT, Foster CS, Grosskreutz CL, Pasquale LR. Postoperative sympathetic ophthalmia. Int Ophthalmol Clin. 2000; 40:69–84.
Article16). Burt RK, Traynor AE, Pope R, et al. Treatment of autoimmune disease by intense immunosuppressive conditioning and autologous hematopoietic stem cell transplantation. Blood. 1998; 92:3505–14.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Remission after Rituximab Therapy in Refractory Myasthenia Gravis
- Primary acquired chronic pure red cell aplasia refractory to standard treatments: remission with rituximab
- Delayed and Long-term Remission of Refractory Hemolytic Anemia in a Child with Systemic Lupus Erythematosus Treated with Rituximab
- A Case of Autoimmune Hemolytic Anemia Treated with Rituximab in a Child
- Steroid Non-responsive Hashimoto's Encephalopathy Improved by Rituximab