Korean J Hematol.
2010 Sep;45(3):188-192. 10.5045/kjh.2010.45.3.188.
The risk factors for herpes zoster in bortezomib treatment in patients with multiple myeloma
- Affiliations
-
- 1Department of Hematology-Oncology, Busan Cancer Center, Pusan National University Hospital Medical Research Institute, Busan, Korea. Hemon@pusan.ac.kr
- 2Department of Hematology-Oncology, School of Medicine, Gyeongsang National University Hospital, Jinju, Korea.
- 3Department of Hematology-Oncology, Kyungpook National University Hospital, Kyungpook National University School of Medicine, Daegu, Korea.
- KMID: 2252061
- DOI: http://doi.org/10.5045/kjh.2010.45.3.188
Abstract
- BACKGROUND
Bortezomib has significant activity in treating multiple myeloma (MM). The risk of herpes zoster (HZ) has been reported to increase significantly with bortezomib treatment, but the predisposing factors for HZ are not clear. This study is a retrospective analysis of the relevant risk factors for HZ in Korean MM patients treated with bortezomib.
METHODS
Sixty-six patients with refractory or relapsed MM who underwent chemotherapy with bortezomib were included in the study. Prophylactic antiviral drugs were not used for treatment. The following parameters were reviewed: age, gender, stage and type of MM, extent of previous treatment, history of HZ, duration from the time of diagnosis to the time of bortezomib treatment initiation, and absolute lymphocyte counts (ALC) at the time of bortezomib treatment initiation.
RESULTS
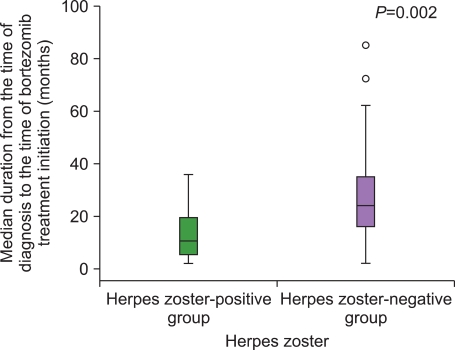
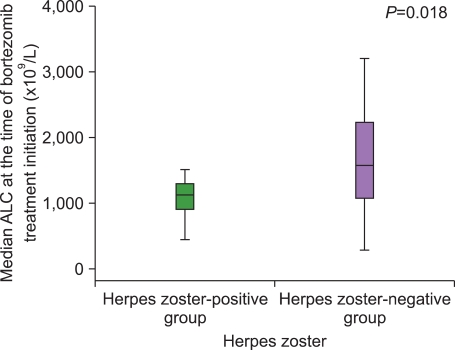
The incidence of HZ was 16.7%. There were no intergroup differences between the HZ-positive and the HZ-negative groups with regard to a history of HZ, number of previous treatments, and exposure to steroids before bortezomib treatment. The median duration from the time of MM diagnosis to the time of bortezomib treatment initiation in the HZ-positive group was significantly shorter than that in the HZ-negative group. The median ALC at the time of bortezomib initiation in the HZ-positive group was significantly lower than that in the HZ-negative group.
CONCLUSION
Bortezomib itself might act as a risk factor for HZ by inhibiting cell-mediated immunity, and patients with low ALC at the time of bortezomib treatment initiation were at greater risk of HZ during bortezomib treatment.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Concerns in assessing risk factors for herpes zoster infection in multiple myeloma patients
Dong-Gun Lee
Korean J Hematol. 2010;45(4):286-286. doi: 10.5045/kjh.2010.45.4.286.
Reference
-
1. Curran MP, McKeage K. Bortezomib: a review of its use in patients with multiple myeloma. Drugs. 2009; 69:859–888. PMID: 19441872.2. Hideshima T, Richardson P, Chauhan D, et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001; 61:3071–3076. PMID: 11306489.3. Richardson PG, Hideshima T, Anderson KC. Bortezomib (PS-341): a novel, first-in-class proteasome inhibitor for the treatment of multiple myeloma and other cancers. Cancer Control. 2003; 10:361–369. PMID: 14581890.
Article4. Chanan-Khan A, Sonneveld P, Schuster MW, et al. Analysis of herpes zoster events among bortezomib-treated patients in the phase III APEX study. J Clin Oncol. 2008; 26:4784–4790. PMID: 18711175.
Article5. Mateos MV, Hernández JM, Hernández MT, et al. Bortezomib plus melphalan and prednisone in elderly untreated patients with multiple myeloma: results of a multicenter phase 1/2 study. Blood. 2006; 108:2165–2172. PMID: 16772605.
Article6. Palumbo A, Ambrosini MT, Benevolo G, et al. Bortezomib, melphalan, prednisone, and thalidomide for relapsed multiple myeloma. Blood. 2007; 109:2767–2772. PMID: 17148584.
Article7. San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008; 359:906–917. PMID: 18753647.
Article8. Kim SJ, Kim K, Kim BS, et al. Bortezomib and the increased incidence of herpes zoster in patients with multiple myeloma. Clin Lymphoma Myeloma. 2008; 8:237–240. PMID: 18765311.
Article9. Zheng W, Wei G, Ye X, et al. Bortezomib in combination with dexamethasone and subsequent thalidomide for newly-diagnosed multiple myeloma: a Chinese experience. Leuk Res. 2009; 33:1615–1618. PMID: 19773080.
Article10. Ohguchi H, Sugawara T, Ishikawa I, et al. A retrospective analysis of bortezomib therapy for Japanese patients with relapsed or refractory multiple myeloma: beta2-microglobulin associated with time to progression. Int J Hematol. 2009; 89:342–347. PMID: 19296199.11. Palumbo A, Gay F, Bringhen S, et al. Bortezomib, doxorubicin and dexamethasone in advanced multiple myeloma. Ann Oncol. 2008; 19:1160–1165. PMID: 18326520.
Article12. Gershon AA, Gershon MD, Breuer J, Levin MJ, Oaklander AL, Griffiths PD. Advances in the understanding of the pathogenesis and epidemiology of herpes zoster. J Clin Virol. 2010; 48(Suppl 1):S2–S7. PMID: 20510263.
Article13. Morison WL. Letter: herpes simplex and herpes zoster in neoplasia. Lancet. 1974; 1:1293. PMID: 4134180.14. Richardson PG, Sonneveld P, Schuster M, et al. Extended follow-up of a phase 3 trial in relapsed multiple myeloma: final time-to-event results of the APEX trial. Blood. 2007; 110:3557–3560. PMID: 17690257.
Article15. Jagannath S, Barlogie B, Berenson J, et al. A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol. 2004; 127:165–172. PMID: 15461622.
Article16. Richardson PG, Barlogie B, Berenson J, et al. phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003; 348:2609–2617. PMID: 12826635.17. Nucci M, Anaissie E. Infections in patients with multiple myeloma in the era of high-dose therapy and novel agents. Clin Infect Dis. 2009; 49:1211–1225. PMID: 19769539.
Article18. Uy GL, Peles S, Fisher NM, Tomasson MH, DiPersio JF, Vij R. Bortezomib prior to autologous transplant in multiple myeloma: effects on mobilization, engraftment, and markers of immune function. Biol Blood Marrow Transplant. 2006; 12(Suppl 1):116.
Article19. Nencioni A, Garuti A, Schwarzenberg K, et al. Proteasome inhibitor-induced apoptosis in human monocyte-derived dendritic cells. Eur J Immunol. 2006; 36:681–689. PMID: 16479541.
Article20. Matsumoto M, Yamada T, Yoshinaga SK, et al. Essential role of NF-kappa B-inducing kinase in T cell activation through the TCR/CD3 pathway. J Immunol. 2002; 169:1151–1158. PMID: 12133934.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Concerns in assessing risk factors for herpes zoster infection in multiple myeloma patients
- A Case of Bilateral Recurrent Herpes Zoster in Multiple Myeloma
- A Case of Acute Pancreatitis Caused by Bortezomib in a Patient with Multiple Myeloma
- Reversible Heart Failure after Bortezomib Treatment in a Patient with Multiple Myeloma
- A Case of Cutaneous Plasmacytoma Treated with Bortezomib and Radiotherapy